Holmium(III) iodide
Chemical compound From Wikipedia, the free encyclopedia
Holmium(III) iodide is an iodide of holmium, with the chemical formula of HoI3. It is used as a component of metal halide lamps.[5]
 | |
| Names | |
|---|---|
| Other names
Holmium iodide Holmium triiodide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.034.050 |
| EC Number |
|
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| HoI3 | |
| Molar mass | 545.6437 g/mol |
| Appearance | Pale-yellow solid[1][2] |
| Density | 5.4 g/cm3[3] |
| Melting point | 994 °C[2] |
| Boiling point | 1300 °C[4] |
| soluble in water[2] | |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H315, H319, H335, H360 | |
| P203, P233, P260, P261, P264, P264+P265, P271, P272, P280, P284, P302+P352, P304+P340, P305+P351+P338, P318, P319, P321, P332+P317, P333+P317, P337+P317, P342+P316, P362+P364, P403, P403+P233, P405, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
Holmium(III) iodide can be obtained by directly reacting holmium and iodine:[4]
- 2 Ho + 3 I2 → 2 HoI3
Holmium(III) iodide can also be obtained via the direct reaction between holmium and mercury(II) iodide:
- 2 Ho + 3 HgI2 → 2 HoI3 + 3 Hg
The mercury produced in the reaction can be removed by distillation.[6]
Holmium(III) iodide hydrate can be converted to the anhydrous form by dehydration with a large excess of ammonium iodide (since the compound is prone to hydrolysis).[4]
Properties
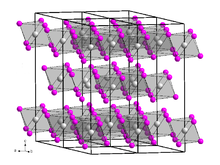
Holmium(III) iodide is a highly hygroscopic substance that dissolves in water.[7][3][2] It forms yellow hexagonal crystals with a crystal structure similar to bismuth(III) iodide.[4] In air, it quickly absorbs moisture and forms hydrates. The corresponding oxide iodide is also readily formed at elevated temperature.[4]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
