Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
Hafnium disulfide is an inorganic compound of hafnium and sulfur. It is a layered dichalcogenide with the chemical formula is HfS2. A few atomic layers of this material can be exfoliated using the standard Scotch Tape technique (see graphene) and used for the fabrication of a field-effect transistor.[4] High-yield synthesis of HfS2 has also been demonstrated using liquid phase exfoliation, resulting in the production of stable few-layer HfS2 flakes.[5] Hafnium disulfide powder can be produced by reacting hydrogen sulfide and hafnium oxides at 500–1300 °C.[6]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Hafnium disulfide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.038.738 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| HfS2 | |
| Molar mass | 246.62 g/mol[1] |
| Appearance | Brown solid |
| Density | 6.03 g/cm3[1] |
| Band gap | ~1.8 eV (indirect)[2] |
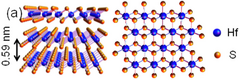
| Structure | |
| hP3, P3m1, No 164[3] | |
a = 0.363 nm, c = 0.584 nm | |
Formula units (Z) |
1 |
| Related compounds | |
Other anions |
Hafnium dioxide |
Other cations |
Tungsten disulfide Molybdenum disulfide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.