Top Qs
Timeline
Chat
Perspective
Ferrole
Class of chemical compounds From Wikipedia, the free encyclopedia
Remove ads
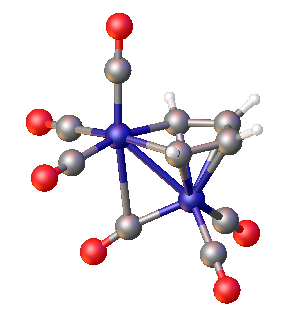
In organoiron chemistry, a ferrole is a type of diiron complex containing the (OC)3FeC4R4 heterocycle that is pi-bonded to a Fe(CO)3 group. These compounds have Fe-Fe bonds (ca. 252 pm) and semi-bridging CO ligands (Fe-C distances = 178, 251 pm). They are typically air-stable, soluble in nonpolar solvents, and red-orange in color.[2]

Remove ads
Synthesis
Ferroles typically arise by the reaction of alkynes with iron carbonyls. Such reactions are known to generate many products, e.g. complexes of cyclopentadienones and para-quinones.[3][4]
Another route involves the desulfurization of thiophenes (SC4R4) by iron carbonyls, shown in the following idealized equation:
- Fe3(CO)12 + SC4R4 → Fe2(CO)6C4R4 + FeS + 6 CO
An unusual route to ferroles involves treatment of Collman's reagent with trimethylsilyl chloride (tms = (CH3)3Si):
- 2 Na2Fe(CO)4 + 4 tmsCl → Fe2(CO)6C4(Otms)4 + 2 CO + 4 NaCl
Remove ads
Reactions
Some ferroles react with tertiary phosphines to give the substituted flyover complex Fe2(CO)5(PR3)(C4R4CO).[5] [6]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads