Loading AI tools
From Wikipedia, the free encyclopedia
The encapsulins are a family of bacterial proteins that serve as the main structural components of encapsulin nanocompartments.[1] There are several different encapsulin proteins, including EncA, which forms the shell, and EncB, EncC, and EncD, which form the core.[1]
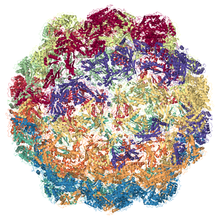 EM structure of Myxococcus xanthus encpasulin protein (EncA) PDB entry 4pt2 | |||||||||
| Identifiers | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Symbol | Linocin_M18 | ||||||||
| Pfam | PF04454 | ||||||||
| InterPro | IPR007544 | ||||||||
| |||||||||
Encapsulins are also used in synthetic biology. They are hard to discover due to their similarity to phage proteins.[2]
Encapsulins were discovered in 1994 as a new class of prokaryotic compartments.[3] Prokaryotic cells usually lack membrane compartments typical for eukaryotes. They instead have numerous protein compartments that are capable of accumulating a large number of molecules.[3]
When protein nanocompartments were discovered in 1994, and later renamed encapsulins, they were found in the supernatant fluid of the Brevibacterium linens culture.[3]
In 2008, encapsulins were recognized as protein-based compartmentalization systems with dedicated functions in cellular organisms.[4]
Encapsulin shells compromise icosahedral complexes (12 vertices, 20 faces, 30 edges) formed as a result of self-assembly of protomers. [3] The shells have diameters between 24 and 42 nm and are defined by the viral HK97-fold of their shell protein. [4] The HK97-fold protomer is roughly triangular and has three conserved domains, the axial domain, peripheral domain, and extended loop.[4]
Encapsulins serve many physiological functions, including catalysis, mineral storage, response to oxidative stress and secondary metabolism. There are ferritin-like encapsulins as well.[2]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.