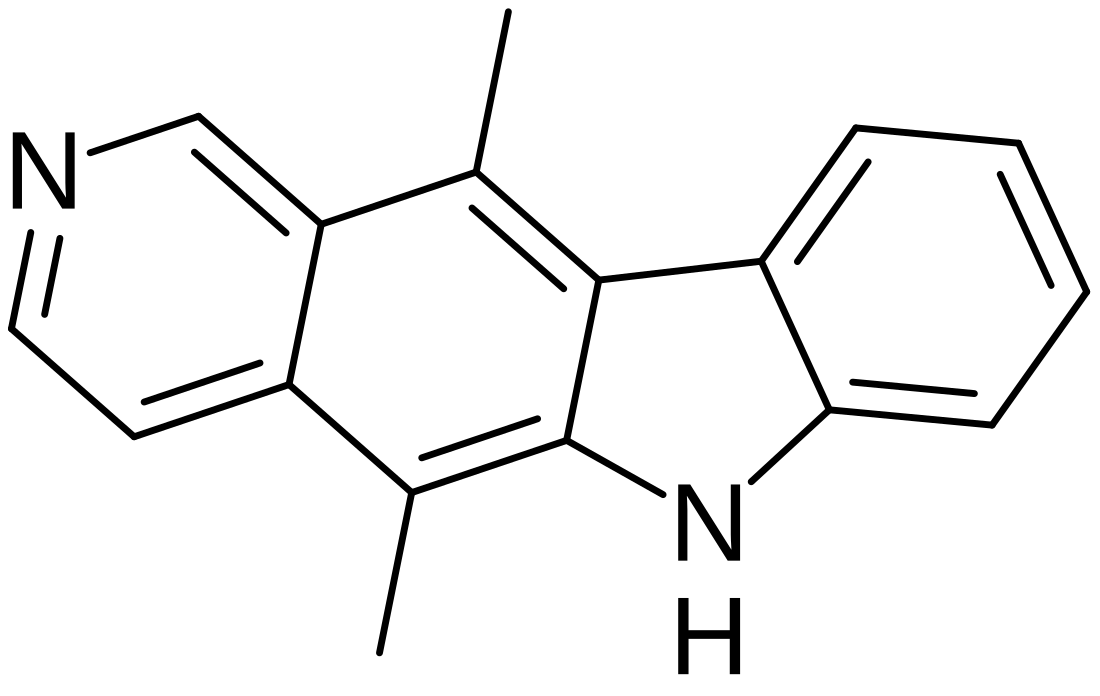
Ellipticine
Chemical compound From Wikipedia, the free encyclopedia
Ellipticine is a tetracyclic alkaloid first extracted from trees of the species Ochrosia elliptica and Rauvolfia sandwicensis,[5][6] which inhibits the enzyme topoisomerase II via intercalative binding to DNA.[7]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
5,11-Dimethyl-6H-pyrido[4,3-b]carbazole | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.007.514 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H14N2 | |
| Molar mass | 246.313 g·mol−1 |
| Appearance | Yellow crystalline powder[1] |
| Density | 1.257±0.06 g/cm3[2] |
| Melting point | 316–318 °C (601–604 °F; 589–591 K)[2] |
| Very low[3] | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
toxic |
| GHS labelling: | |
 [4] [4] | |
| H301[4] | |
| P264, P270, P301+P310, P321, P330, P405, P501[4] | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Natural occurrence and synthesis
Ellipticine is an organic compound present in several trees within the genera Ochrosia, Rauvolfia, Aspidosperma, and Apocynaceae.[8] It was first isolated from Ochrosia elliptica Labill., a flowering tree native to Australia and New Caledonia which gives the alkaloid its name, in 1959,[5] and synthesised by Robert Burns Woodward later the same year.[6]
Biological activity
Ellipticine is a known intercalator, capable of entering a DNA strand between base pairs. In its intercalated state, ellipticine binds strongly[9] and lies parallel to the base pairs,[10] increasing the superhelical density of the DNA.[11] Intercalated ellipticine binds directly to topoisomerase II, an enzyme involved in DNA replication,[12] inhibiting the enzyme and resulting in powerful antitumour activity.[10] In clinical trials, ellipticine derivatives have been observed to induce remission of tumour growth, but are not used for medical purposes due to their high toxicity; side effects include nausea and vomiting, hypertension, cramp, pronounced fatigue, mouth dryness, and mycosis of the tongue and oesophagus.[13]
Further DNA damage results from the formation of covalent DNA adducts following enzymatic activation of ellipticine by with cytochromes P450 and peroxidases, meaning that ellipticine is classified as a prodrug.[14]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.