Disulfur difluoride
Chemical compound From Wikipedia, the free encyclopedia
Disulfur difluoride is an inorganic compound with the chemical formula S2F2. It is a halide of sulfur.
 | |||
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
fluorosulfanyl thiohypofluorite | |||
| Other names
Difluorodisulfane[1] | |||
| Identifiers | |||
3D model (JSmol) |
|||
| ChemSpider | |||
PubChem CID |
|||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| S2F2 | |||
| Molar mass | 102.127 g/mol | ||
| Melting point | −133 °C (−207 °F; 140 K) | ||
| Boiling point | 15 °C (59 °F; 288 K) | ||
| Related compounds | |||
Related compounds |
|||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
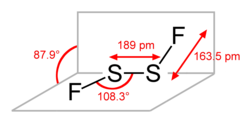
Structure
Disulfur difluoride has a chain structure F−S−S−F. The angle between the Fa−S−S and S−S−Fb planes is 87.9°, while the angles of Fa−S−S and S−S−Fb are equivalent, and are equal to 108.3°. Both S−F bonds are equivalent and their length is 163.5 pm, while the length of the S−S bond is 189 pm. This structure is referred to as gauche, and is similar to H2O2.
There is a branched isomer of disulfur difluoride, thiothionyl fluoride, with the structure S=SF2.
Synthesis
Silver(II) fluoride can fluorinate sulfur in a strictly dry container at 125 °C (257 °F; 398 K), and the reaction produces FS−SF:[2]
Reactions
Disulfur difluoride undergoes intramolecular rearrangement in the presence of fluorides of alkali metals, yielding the isomer S=SF2:[3]
- FS−SF → S=SF2
- Decomposing to sulfur tetrafluoride and sulfur when heated to 180 °C:
- 2 S2F2 → SF4 + 3 S
- Reacting with sulfuric acid at 80 °C:
- S2F2 + 3 H2SO4 → 5 SO2 + 2 HF + 2 H2O
- Reacting with sodium hydroxide:
- Reacting with oxygen at high pressure, using nitrogen dioxide as a catalyst:
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.


