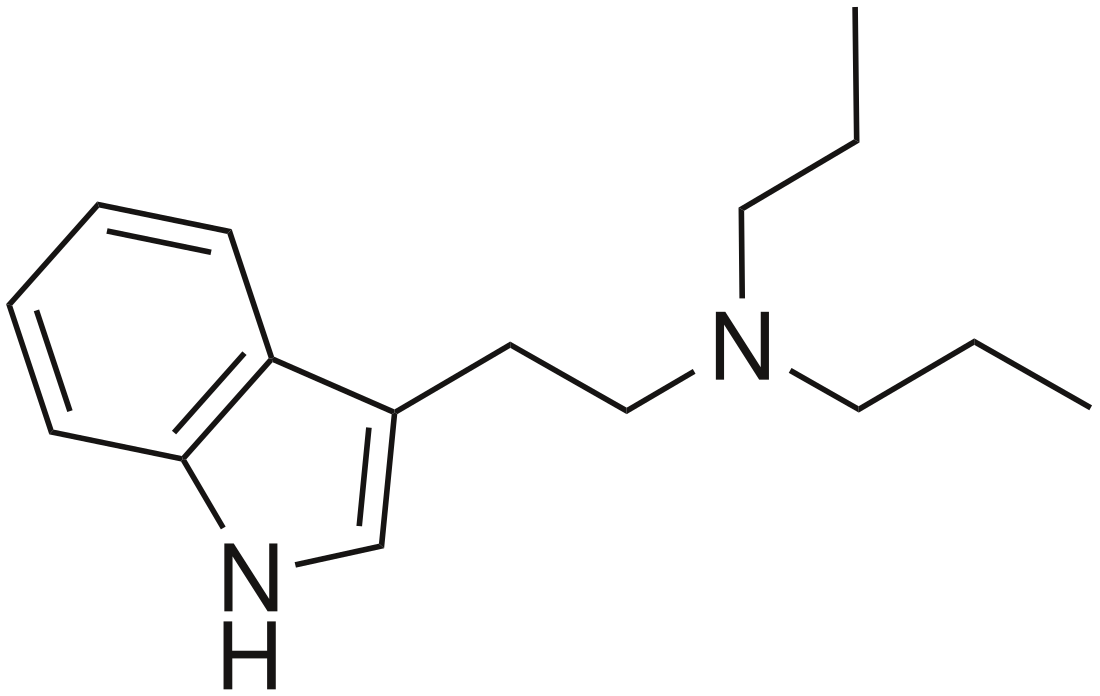
Dipropyltryptamine
Chemical compound From Wikipedia, the free encyclopedia
N,N-Dipropyltryptamine (DPT) is a psychedelic drug and entheogen belonging to the tryptamine family.[1] Use as a designer drug has been documented by law enforcement officials since as early as 1968.[2] However, potential therapeutic use was not investigated until the 1970s.[3] It is found either as a crystalline hydrochloride salt or as an oily or crystalline base. It has not been found to occur endogenously. It is a close structural homologue of dimethyltryptamine and diethyltryptamine.
 | |
 | |
| Clinical data | |
|---|---|
| Other names | DPT; N,N-Dipropyltryptamine |
| Routes of administration | Oral, inhalation (smoking), intravenous or intramuscular injection[1] |
| Drug class | Serotonin receptor agonist; Serotonin 5-HT2A receptor agonist; Serotonergic psychedelic; Hallucinogen |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Onset of action | Injection: 10–15 minutes[1] |
| Duration of action | 2–4 hours[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H24N2 |
| Molar mass | 244.382 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 174.5 to 178 °C (346.1 to 352.4 °F) |
| |
| |
| (verify) | |
Use and effects
Doses ranges of DPT of 100 to 250 mg (but up to 500 mg) orally, 100 mg smoked, 15 to 125 mg intramuscularly, and 12 to 36 mg intravenously have been described.[1][4] Its duration is 2 to 4 hours orally but can last up to 12 hours with high doses.[1]
While DPT is chemically similar to dimethyltryptamine (DMT), its psychoactive effects have been said to be markedly different.[5] On the other hand, others have reported similarities to DMT, for instance in terms of intensity.[1]
Side effects
Side effects of DET may include nausea, numbness of the tongue or throat, pupil dilation, increased heart rate, dizziness, anxiety, panic, confusion, paranoia, delusions, and seizures (uncommon).[citation needed]
The use of DPT has been implicated in at least one death due to seizures,[6] although details are lacking and the drug has not officially been established as the sole cause of death.
Interactions
Pharmacology
Summarize
Perspective
| Target | Affinity (Ki, nM) | Species |
|---|---|---|
| 5-HT1A | 31.8–1,641 (Ki) 274–>10,000 (EC50) 99% (Emax) | Human Human |
| 5-HT1B | 854–8,081 (Ki) 1,210 (EC50) | Human Human |
| 5-HT1D | 619 | Human |
| 5-HT1E | 2,338 | Human |
| 5-HT2A | 3.0–2,579 (Ki) 26.1–943 (EC50) 85–97% (Emax) | Human Human Human |
| 5-HT2B | 42 | Human |
| 5-HT2C | 281–3,500 (Ki) 444 (EC50) 93% (Emax) | Human |
| 5-HT3 | >10,000 | Human |
| 5-HT4 | ND | ND |
| 5-HT5A | 4,373 | Human |
| 5-HT6 | 4,543 | Human |
| 5-HT7 | 284 | Human |
| D1 | >10,000 | Human |
| D2 | 9,249 | Human |
| D3 | 1,361 | Human |
| D4 | 2,014 | Human |
| D5 | >10,000 | Human |
| α1A | 881 | Human |
| α1B | 443 | Human |
| α1D | ND | ND |
| α2A | 458 | Human |
| α2B | 339 | Human |
| α2C | 514 | Human |
| β1–β2 | >10,000 | Human |
| H1 | 125 | Human |
| H2–H4 | >10,000 | Human |
| M1–M5 | >10,000 | Human |
| I1 | 340 | Human |
| σ1 | 397 | Human |
| σ2 | 2,917 | Human |
| SERT | 157 (Ki) 157–23,000 (IC50) >100,000 (EC50) | Human Human Rat |
| NET | >10,000 (Ki) 2,900–3,202 (IC50) >100,000 (EC50) | Human Human Rat |
| DAT | 1,500 (Ki) 2,218–9,100 (IC50) >100,000 (EC50) | Human Human Rat |
| Notes: The smaller the value, the more avidly the drug binds to the site. Refs: [7][8][9][10][11][12][13] | ||
Studies on rodents have found that the effectiveness with which a selective 5-HT2A receptor antagonist blocks the behavioral actions of this compound strongly suggests that the 5-HT2A receptor is an important site of action for DPT, but the modulatory actions of a 5-HT1A receptor antagonist also imply a 5-HT1A-mediated component to the actions of DPT.[14]
DPT produces the head-twitch response, a behavioral proxy of psychedelic-like effects, in rodents.[4]
Chemistry

DPT changes Ehrlich's reagent violet and causes the marquis reagent to turn yellow.[15]
History
DPT was first described in the scientific literature by 1959.[16][17][18]
Society and culture
Religious use
DPT is used as a religious sacrament by the Temple of the True Inner Light, a New York City offshoot of the Native American Church. The Temple believes DPT and other entheogens are physical manifestations of God.[19]
Legal status
Sweden
DPT is illegal in Sweden as of 26 January 2016.[20]
United Kingdom
DPT is a Class A drug in the United Kingdom, making it illegal to possess or distribute.
United States
DPT is not scheduled at the federal level in the United States,[21] but it could be considered an analog of 5-MeO-DiPT, DMT, or DET, in which case purchase, sale, or possession could be prosecuted under the Federal Analogue Act.
Florida
"DPT (N,N-Dipropyltryptamine)" is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess in Florida.[22]
Maine
DPT is a Schedule I controlled substance in the state of Maine making it illegal to buy, sell, or possess in Maine.
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.