Diethylthiambutene
Chemical compound From Wikipedia, the free encyclopedia
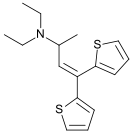
Diethylthiambutene (Thiambutene, Themalon, Diethibutin, N,N-Diethyl-1-methyl-3,3-di-2-thienylallylamine) is an opioid analgesic drug developed in the 1950s[2] which was mainly used as an anesthetic in veterinary medicine and continues, along with the other two thiambutenes dimethylthiambutene and ethylmethylthiambutene to be used for this purpose, particularly in Japan.[3][4] It is now under international control under Schedule I of the UN Single Convention On Narcotic Drugs 1961, presumably due to high abuse potential, although little more information is available. It is listed under Schedule I of the US Controlled Substances Act as a Narcotic and has an ACSCN of 9616 with zero annual manufacturing quota as of 2013.
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C16H21NS2 |
| Molar mass | 291.47 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 152 to 153 °C (306 to 307 °F) |
| |
| |
| (what is this?) (verify) | |
Synthesis

The conjugate addition of diethylamine [109-89-7] to ethyl crotonate [623-70-1] [10544-63-5] (1) gives ethyl 3-(diethylamino)butanoate, CID:10679145 (2). Addition of two equivalents of 2-thienyllithium to the ester gives the tertiary alcohol [94094-46-9] (4'). The dehydration of this then completes the synthesis of diethylthiambutene (5').
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
