Dibenzylideneacetone
Chemical compound From Wikipedia, the free encyclopedia
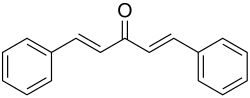
Dibenzylideneacetone or dibenzalacetone, often abbreviated dba, is an organic compound with the formula C17H14O. It is a pale-yellow solid insoluble in water, but soluble in ethanol.
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(1E,4E)-1,5-Diphenylpenta-1,4-dien-3-one | |
| Other names
Dibenzalacetone | |
| Identifiers | |
| |
3D model (JSmol) |
|
| ChEMBL |
|
| ChemSpider | |
| ECHA InfoCard | 100.126.050 |
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C17H14O | |
| Molar mass | 234.29 g/mol |
| Appearance | Yellow solid |
| Melting point | |
| Boiling point | 130 °C (266 °F; 403 K) (cis, cis isomer)[1] |
| Insoluble | |
| Solubility in other solvents | Soluble in acetone and chloroform, slightly soluble in ethanol. |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritant |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
It was first prepared in 1881 by the German chemist Rainer Ludwig Claisen (1851–1930) and the Swiss chemist Charles-Claude-Alexandre Claparède (14 April 1858 – 1 November 1913).[2][3][4]
Preparation
The trans,trans isomer can be prepared in high yield and purity by condensation of benzaldehyde and acetone with sodium hydroxide in a water/ethanol medium followed by recrystallization.[5]
This reaction, which proceeds via the intermediacy of benzylideneacetone, is often performed in organic chemistry classes,[6] and is called Claisen-Schmidt condensation.
Reactions and derivatives
Prolonged exposure to sunlight initiates [2+2] cycloadditions, converting it to a mixture of dimeric and trimeric cyclobutane cycloadducts.[7]
Uses
Dibenzylideneacetone is used as a component in sunscreens and as a ligand in organometallic chemistry.
For example, it is a component of the catalyst tris(dibenzylideneacetone)dipalladium(0). It is a labile ligand that is easily displaced by triphenylphosphine, hence it serves a useful entry point into palladium(0) chemistry.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.

