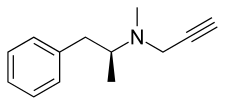
d-Deprenyl, also known as or dextro-N-propargyl-N-methylamphetamine, is an MAO-B inhibitor that metabolizes into d-amphetamine and d-methamphetamine and is therefore also a norepinephrine–dopamine releasing agent.[1][2][3][4][5] It is one of the two enantiomers of deprenyl and is the opposite enantiomer of l-deprenyl (selegiline).
 | |
| Clinical data | |
|---|---|
| Other names | dextro-N-propargyl-N-methylamphetamine |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C13H17N |
| Molar mass | 187.286 g·mol−1 |
| 3D model (JSmol) | |
| Chirality | Dextrorotatory enantiomer |
| |
| |
| | |
l-Deprenyl, also an MAO-B inhibitor, metabolizes to l-amphetamine and l-methamphetamine, which are both norepinephrine releasing agents. In contrast, d-deprenyl additionally has dopaminergic effects and has been found to be reinforcing in scientific research, whereas l-deprenyl is not known to have any appreciable psychological reinforcement.[6][7]
In addition to its actions as an MAO-B inhibitor and NDRA, d-deprenyl has been found to bind with high affinity to the σ1 receptor (Ki = 79 nM) similarly to various other amphetamine derivatives.[8][9] Its l-isomer, selegiline, binds with 3.5-fold lower affinity in comparison.[8][9]
See also
References
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.
