Cumene hydroperoxide
Aromatic organic chemical compound From Wikipedia, the free encyclopedia
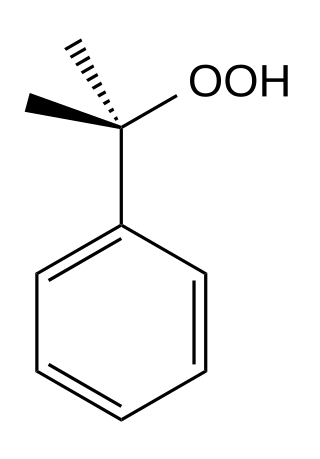
Cumene hydroperoxide is the organic compound with the formula C6H5C(CH3)2OOH; this oily liquid is classified as an organic hydroperoxide.[2] Products of decomposition of cumene hydroperoxide are methylstyrene, acetophenone, and 2-phenylpropan-2-ol.[3]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
2-Phenylpropane-2-peroxol | |
| Other names
Cumyl hydroperoxide CHP | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.001.141 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H12O2 | |
| Molar mass | 152.193 g·mol−1 |
| Appearance | Colorless to pale yellow liquid |
| Density | 1.02 g/cm3 |
| Melting point | −9 °C (16 °F; 264 K) |
| Boiling point | 153 °C (307 °F; 426 K) |
| 1.5 g/100 mL | |
| Vapor pressure | 14 mmHg, at 20 °C |
| Hazards | |
| GHS labelling: | |
     | |
| Danger | |
| H242, H302, H312, H314, H331, H373, H411 | |
| P220, P261, P273, P280, P305+P351+P338, P310 | |
| NFPA 704 (fire diamond) | |
| Flash point | 57 °C (135 °F; 330 K) |
| Safety data sheet (SDS) | sigmaaldrich.com |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
It is produced by treatment of cumene with oxygen, an autoxidation. At temperatures >100 °C, oxygen is passed through liquid cumene:[4]
- C
6H
5CH(CH
3)
2 + O2 → C
6H
5C(CH
3)
2OOH
Dicumyl peroxide is a side product.
Applications
Cumene hydroperoxide is an intermediate in the cumene process for producing phenol and acetone from benzene and propene.
Cumene hydroperoxide is a radical initiator for production of acrylates.[5]
Cumene hydroperoxide is involved as an organic peroxide in the production of propylene oxide by the oxidation of propene. This technology was commercialized by Sumitomo Chemical.[6]
The oxidation by cumene hydroperoxide of propene affords propylene oxide and the byproduct 2-phenylpropan-2-ol. The reaction follows this stoichiometry:
- CH
3CHCH
2 + C
6H
5C(CH
3)
2OOH → CH
3CHCH
2O + C
6H
5C(CH
3)
2OH
Dehydrating and hydrogenating cumyl alcohol recycles the cumene.
Safety
Cumene hydroperoxide, like all organic peroxides, is potentially explosive. It is also toxic, corrosive and flammable as well as a skin-irritant.[7]
References
Related terms
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.