Coenzyme B
Chemical compound From Wikipedia, the free encyclopedia
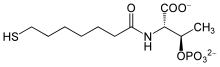
Coenzyme B is a coenzyme required for redox reactions in methanogens. The full chemical name of coenzyme B is 7-mercaptoheptanoylthreoninephosphate.[1] The molecule contains a thiol, which is its principal site of reaction.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-[(7-mercapto-1-oxoheptyl)amino]-3-phosphonooxybutanoic acid | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C 11H 22NO 7PS | |
| Molar mass | 343.333641 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Coenzyme B reacts with 2-methylthioethanesulfonate (methyl-Coenzyme M, abbreviated CH
3–S–CoM), to release methane in methanogenesis:[2]
- CH
3–S–CoM + HS–CoB → CH
4 + CoB–S–S–CoM
This conversion is catalyzed by the enzyme methyl coenzyme M reductase, which contains cofactor F430 as the prosthetic group.
A related conversion that utilizes both HS-CoB and HS-CoM is the reduction of fumarate to succinate, catalyzed by fumarate reductase:[3]
- HS–CoM + HS–CoB + −O
2CCH=CHCO−
2 → −O
2CCH
2–CH
2CO−
2 + CoB–S–S–CoM
Importance of coenzyme B in methanogenesis
Coenzyme B is an important component in the terminal step of methane biogenesis.[4] It acts as a two electron-donor to reduce coenzyme M (methyl-coenzyme) into two molecules a methane and a heterodisulfide.[5] Two separate experiments that were performed, one with coenzyme B and other without coenzyme B, indicated that using coenzyme B before the formation of the methane molecule, results in a more efficient and consistent bond cleavage.[6]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
