Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
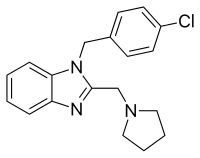
Clemizole, sold under the brand names Allercur and Histacur, is a histamine H1 receptor antagonist of the benzimidazole group described as an antihistamine, antipruritic, and sedative which is no longer marketed.[1][2][3][4]
 | |
| Clinical data | |
|---|---|
| Trade names | Allercur, Histacur |
| Other names | EPX-100 |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.006.486 |
| Chemical and physical data | |
| Formula | C19H20ClN3 |
| Molar mass | 325.84 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
It is also a serotonin receptor agonist and is being studied for the potential treatment of Dravet syndrome, Lennox-Gastaut syndrome, and epilepsy under the development code name EPX-100.[5][4][6]
The drug was first described in the scientific literature by 1952.[1] Its serotonin receptor agonist and anticonvulsant properties were discovered in 2017.[5][4]
Benzimidazoles substituted with an alkylamine at position 2 have a venerable history as H1 antihistaminic agents. The standard starting material for many benzimidazoles consists of phenylenediamine, or its derivatives.

Reaction of that compound with chloroacetic acid can be rationalized by invoking initial formation of the chloromethyl amide. Imide formation with the remaining free amino group closes the ring to afford 2-chloromethyl benzimidazole (3). Displacement of halogen with pyrrolidine affords the alkylation product. The proton on the fused imidazole nitrogen is then removed by reaction with sodium hydride. Treatment of the resulting anion with α,4-dichlorotoluene gives the H1 antihistaminic agent clemizole (5).
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.