Clascoterone
Chemical compound From Wikipedia, the free encyclopedia
Clascoterone, sold under the brand name Winlevi, is an antiandrogen medication which is used topically in the treatment of acne.[5][6][7] It is also under development in a higher concentration for the treatment of androgen-dependent scalp hair loss, under the brand name Breezula.[6] The medication is used as a cream by application to the skin, for instance the face and scalp.[7]
 | |
| Clinical data | |
|---|---|
| Trade names | Winlevi |
| Other names | CB-03-01; Breezula; 11-Deoxycortisol 17α-propionate; 17α-(Propionyloxy)- deoxycorticosterone; 21-Hydroxy-3,20-dioxopregn-4-en-17-yl propionate |
| AHFS/Drugs.com | Monograph |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Topical |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.210.810 |
| Chemical and physical data | |
| Formula | C24H34O5 |
| Molar mass | 402.531 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Clascoterone is an antiandrogen, or antagonist of the androgen receptor (AR), the biological target of androgens such as testosterone and dihydrotestosterone.[8][9] It shows minimal systemic absorption when applied to skin.[7]
The medication, developed by Cassiopea and Intrepid Therapeutics,[6] was approved by the US Food and Drug Administration (FDA) for acne in August 2020.[10][11] The U.S. Food and Drug Administration (FDA) considers it to be a first-in-class medication.[12]
Medical uses
Clascoterone is indicated for the topical treatment of acne vulgaris in people aged twelve years of age and older.[5][13]
Two large phase III randomized controlled trials evaluated the effectiveness of clascoterone for the treatment of acne over a period of 12 weeks.[5][13][14] Clascoterone decreased acne symptoms by about 8 to 18% more than placebo.[5][14] The defined treatment success endpoint was achieved in about 18 to 20% of individuals with clascoterone relative to about 7 to 9% of individuals with placebo.[5][13][14] The comparative effectiveness of clascoterone between males and females was not described.[5][14]
A small pilot randomized controlled trial in 2011 found that clascoterone cream decreased acne symptoms to a similar or significantly greater extent than tretinoin 0.05% cream.[13][15] No active comparator was used in the phase III clinical trials of clascoterone for acne.[13] Hence, it's unclear how clascoterone compares to other therapies used in the treatment of acne.[13]
Available forms
Clascoterone is available in the form of a 1% (10 mg/g) cream for topical use.[5]
Side effects
The effects of local skin reactions with clascoterone were similar to placebo in two large phase III randomized controlled trials.[5][14] Suppression of the hypothalamic–pituitary–adrenal axis (HPA axis) may occur during clascoterone therapy in some individuals due to its cortexolone metabolite.[5][13] HPA axis suppression as measured by the cosyntropin stimulation test was observed to occur in 3 of 42 (7%) of adolescents and adults using clascoterone for acne.[5][13] HPA axis function returned to normal within 4 weeks following discontinuation of clascoterone.[5][13] Hyperkalemia (elevated potassium levels) occurred in 5% of clascoterone-treated individuals and 4% of placebo-treated individuals.[5]
Pharmacology
Summarize
Perspective
Pharmacodynamics
Clascoterone is a steroidal antiandrogen, or antagonist of the androgen receptor (AR), the biological target of androgens such as testosterone and dihydrotestosterone (DHT).[5][8][9] In a bioassay, the topical potency of the medication was greater than that of progesterone, flutamide, and finasteride and was equivalent to that of cyproterone acetate.[16] Likewise, it is significantly more efficacious as an antiandrogen than other AR antagonists such as enzalutamide and spironolactone in scalp dermal papilla cells and sebocytes in vitro.[9]
Pharmacokinetics
Steady-state levels of clascoterone occur within 5 days of twice daily administration.[5] At a dosage of 6 g clascoterone cream applied twice daily, maximal circulating levels of clascoterone were 4.5 ± 2.9 ng/mL, area-under-the-curve levels over the dosing interval were 37.1 ± 22.3 h*ng/mL, and average circulating levels of clascoterone were 3.1 ± 1.9 ng/mL.[5] In rodents, clascoterone has been found to possess strong local antiandrogenic activity, but negligible systemic antiandrogenic activity when administered via subcutaneous injection.[16] Along these lines, the medication is not progonadotropic in animals.[16]
The plasma protein binding of clascoterone is 84 to 89% regardless of concentration.[5]
Clascoterone is rapidly hydrolyzed into cortexolone (11-deoxycortisol) and this compound is a possible primary metabolite of clascoterone based on in-vitro studies in human liver cells.[5][13] During treatment with clascoterone, cortexolone levels were detectable and generally below or near the low limit of quantification (0.5 ng/mL).[5] Clascoterone may also produce other metabolites, including conjugates.[5]
The elimination of clascoterone has not been fully characterized in humans.[5]
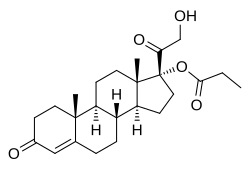
Chemistry
Clascoterone, also known as cortexolone 17α-propionate or 11-deoxycortisol 17α-propionate, as well as 17α,21-dihydroxyprogesterone 17α-propionate or 17α,21-dihydroxypregn-4-en-3,20-dione 17α-propionate, is a synthetic pregnane steroid and a derivative of progesterone and 11-deoxycortisol (cortexolone).[17] It is specifically the C17α propionate ester of 11-deoxycortisol.[16]
An analogue of clascoterone is 9,11-dehydrocortexolone 17α-butyrate (CB-03-04).[18]Corticosteroids related to clascoterone, for instance cortisone acetate and prednisolone acetate, show antiandrogenic activity in animals similarly to clascoterone.[19]
History
Summarize
Perspective
C17α esters of 11-deoxycortisol were unexpectedly found to possess antiandrogenic activity.[16] Clascoterone, also known as cortexolone 17α-propionate, was selected for development based on its optimal drug profile.[16] The medication was approved by the US Food and Drug Administration (FDA) for the treatment of acne in August 2020.[10]
The FDA approved clascoterone based on evidence from two clinical trials (Trial 1/NCT02608450 and Trial 2/NCT02608476) of 1,440 participants 9 to 58 years of age with acne vulgaris.[20] The trials were conducted at 99 sites in the United States, Poland, Romania, Bulgaria, Ukraine, Georgia, and Serbia.[20] Participants applied clascoterone or vehicle (placebo) cream twice daily for 12 weeks.[20] Neither the participants nor the health care providers knew which treatment was being given until after the trial was completed.[20] The benefit of clascoterone in comparison to placebo was assessed after 12 weeks of treatment using the Investigator's Global Assessment (IGA) score that measures the severity of disease (on a scale from 0 to 4) and a decrease in the number of acne lesions.[20]
In October 2023, Cosmo Pharmaceuticals announced it had submitted Winlevi to the European Medicines Agency (EMA) for centralized marketing authorization in order to market Winlevi in the EU.[21]
Society and culture
Names
Clascoterone is the international nonproprietary name and the United States Adopted Name.[17][22]
Research
Clascoterone has been suggested as a possible treatment for hidradenitis suppurativa (acne inversa), an androgen-dependent skin condition.[23]
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
