Cinnamaldehyde
Chemical compound From Wikipedia, the free encyclopedia
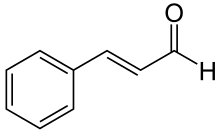
Cinnamaldehyde is an organic compound with the formula C9H8O or C6H5CH=CHCHO. Occurring naturally as predominantly the trans (E) isomer, it gives cinnamon its flavor and odor.[1] It is a phenylpropanoid that is naturally synthesized by the shikimate pathway.[2] This pale yellow, viscous liquid occurs in the bark of cinnamon trees and other species of the genus Cinnamomum. It is an essential oil. The bark of cinnamon tree contains high concentrations of cinnamaldehyde.[3]
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(2E)-3-Phenylprop-2-enal | |
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| 1071571 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.111.079 |
| EC Number |
|
| KEGG | |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C9H8O | |
| Molar mass | 132.16 g/mol |
| Appearance | Yellow oil |
| Odor | Pungent, cinnamon-like |
| Density | 1.0497 g/mL |
| Melting point | −7.5 °C (18.5 °F; 265.6 K) |
| Boiling point | 248 °C (478 °F; 521 K) |
| Slightly soluble | |
| Solubility |
|
| −7.48×10−5 cm3/mol | |
Refractive index (nD) |
1.6195 |
| Hazards | |
| GHS labelling: | |
 | |
| Warning | |
| H315, H317, H319, H335 | |
| P261, P264, P271, P272, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P333+P313, P337+P313, P362, P363, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | 71 °C (160 °F; 344 K) |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
3400 mg/kg (rat, oral) |
| Related compounds | |
Related compounds |
Cinnamic acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Structure and synthesis
Summarize
Perspective
Cinnamaldehyde was isolated from cinnamon essential oil in 1834 by Jean-Baptiste Dumas and Eugène-Melchior Péligot[4] and synthesized in the laboratory by the Italian chemist Luigi Chiozza in 1854.[5]
The natural product is trans-cinnamaldehyde. The molecule consists of a benzene ring attached to an unsaturated aldehyde. Cinnamaldehyde is an α,β-unsaturated carbonyl compound. Its color is due to the π → π* transition: increased conjugation in comparison with acrolein shifts this band towards the visible.[6]
Biosynthesis

Cinnamaldehyde is biosynthesized from phenylalanine.[7] Deamination of L-phenylalanine into cinnamic acid is catalyzed by phenylalanine ammonia lyase (PAL).[8][9] PAL catalyzes this reaction by a non-oxidative deamination. This deamination relies on the MIO prosthetic group of PAL.[10] PAL gives rise to trans-cinnamic acid. In the second step, 4-coumarate–CoA ligase (4CL) converts cinnamic acid to cinnamoyl-CoA by an acid–thiol ligation.[8] 4CL uses ATP to catalyze the formation of cinnamoyl-CoA.[11] 4CL effects this reaction in two steps.[12] 4CL forms a hydroxycinnamate–AMP anhydride, followed by a nucleophile attack on the carbonyl of the acyl adenylate.[13] Finally, Cinnamoyl-CoA is reduced by NADPH catalyzed by CCR (cinnamoyl-CoA reductase) to form cinnamaldehyde.[8][14]
Preparation
Several methods of laboratory synthesis exist. The compound can be prepared from related compounds such as cinnamyl alcohol. An early synthesis involved the aldol condensation of benzaldehyde and acetaldehyde.[15] Cinnamaldehyde can also be obtained from the steam distillation of the oil of cinnamon bark.
Applications
As a flavorant
The most obvious application for cinnamaldehyde is as flavoring in chewing gum, ice cream, candy, e-liquid and beverages; use levels range from 9 to 4,900 parts per million (ppm) (that is, less than 0.5%). It is also used in some perfumes of natural, sweet, or fruity scents. Almond, apricot, butterscotch, and other aromas may partially employ the compound for their pleasant smells. Cinnamaldehyde can be used as a food adulterant; powdered beechnut husk aromatized with cinnamaldehyde can be marketed as powdered cinnamon.[16] Some breakfast cereals contain as much as 187 ppm cinnamaldehyde.[17]
As an agrichemical
Cinnamaldehyde has been tested as a safe and effective insecticide against mosquito larvae.[18] A concentration of 29 ppm of cinnamaldehyde kills half of Aedes aegypti mosquito larvae in 24 hours.[19] Trans-cinnamaldehyde works as a potent fumigant and practical repellant for adult mosquitos.[20] It also has antibacterial and antifungal properties.[21][22]
Miscellaneous uses
Cinnamaldehyde is a corrosion inhibitor for steel and other alloys. It is believed to form a protective film on the metal surface.[23]
Derivatives
Numerous derivatives of cinnamaldehyde are commercially useful. Dihydrocinnamyl alcohol (3-phenylpropanol) occurs naturally but is produced by double hydrogenation of cinnamaldehyde. It has the fragrances of hyacinth and lilac. Cinnamyl alcohol similarly occurs naturally and has the odor of lilac but can be also produced starting from cinnamaldehyde.[24] Dihydrocinnamaldehyde is produced by the selective hydrogenation of the alkene subunit. α-Amylcinnamaldehyde and α-hexylcinnamaldehyde are important commercial fragrances, but they are not prepared from cinnamaldehyde.[16] Hydrogenation of cinnamaldehyde, if directed to the alkene, gives hydrocinnamaldehyde.
Toxicology
Cinnamaldehyde is used in agriculture because of its low toxicity, but it is a skin irritant.[25] Cinnamaldehyde may cause allergic contact stomatitis in sensitised individuals, however allergy to the compound is believed to be uncommon.[26]
Cinnamaldehyde can contain traces of styrene, which arises during storage or transport. Styrene especially forms in high humidity and high temperatures.[27]
DNA repair
Cinnamaldehyde is a dietary antimutagen that effectively inhibits both induced and spontaneous mutations.[28] Experimental evidence indicates that cinnamaldehyde induces a type of DNA damage in the bacterium Escherichia coli and in human cells that elicits recombinational DNA repair that then reduces spontaneous mutations.[28][29] In mice, X-ray–induced chromosome aberrations were reduced when cinnamaldehyde was given orally to the mice after X-ray irradiation,[30] perhaps due to cinnamaldehyde-stimulated DNA repair.
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.

