Chlorine pentafluoride
Chemical compound From Wikipedia, the free encyclopedia
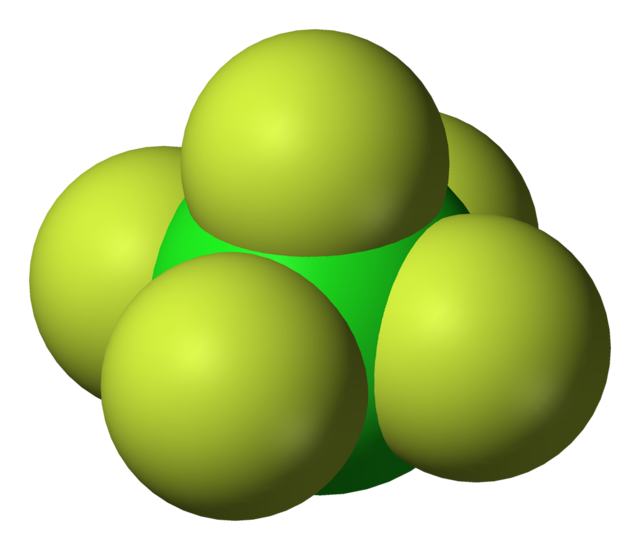
Chlorine pentafluoride is an interhalogen compound with formula ClF5. This colourless gas is a strong oxidant that was once a candidate oxidizer for rockets. The molecule adopts a square pyramidal structure with C4v symmetry,[2] as confirmed by its high-resolution 19F NMR spectrum.[3] It was first synthesized in 1963.[4]
| |||
 | |||
| Identifiers | |||
|---|---|---|---|
3D model (JSmol) |
|||
| ChemSpider | |||
| ECHA InfoCard | 100.033.734 | ||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| ClF5 | |||
| Molar mass | 130.445 g/mol | ||
| Appearance | colorless gas | ||
| Odor | sweet | ||
| Density | 4.5 kg/m3 (g/L) | ||
| Melting point | −103 °C (−153 °F; 170 K) | ||
| Boiling point | −13.1 °C (8.4 °F; 260.0 K) | ||
| Hydrolyzes | |||
| Structure | |||
| Square pyramidal | |||
| Thermochemistry | |||
Std molar entropy (S⦵298) |
310.73 J/(mol·K) | ||
Std enthalpy of formation (ΔfH⦵298) |
−238.49 kJ/mol | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
Main hazards |
Fatal if inhaled. Causes permanent blindness and severe skin and respiratory tract burns.[1] | ||
| GHS labelling: | |||
    | |||
| Danger | |||
| H270, H280, H314, H330 | |||
| P220, P244, P260, P264, P271, P280, P284, P301+P330+P331, P302+P361+P354, P304+P340, P305+P354+P338, P316, P320, P321, P363, P370+P376, P403, P403+P233, P405, P410+P403, P501 | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Preparation
Some of the earliest research on the preparation was classified.[5][6] It was first prepared by fluorination of chlorine trifluoride at high temperatures and high pressures:[5]
NiF2 catalyzes this reaction.[7]
Certain metal fluorides, M+[ClF4]−, e.g. K+[ClF4]− (potassium tetrafluorochlorate(III)), Rb+[ClF4]− (rubidium tetrafluorochlorate(III)), Cs+[ClF4]− (caesium tetrafluorochlorate(III)), react with F2 to produce ClF5 and the corresponding alkali metal fluoride.[6]
Reactions
In a highly exothermic reaction, ClF5 reacts with water to produce chloryl fluoride and hydrogen fluoride:[8]
- ClF5 + 2 H2O → ClO2F + 4 HF
It is also a strong fluorinating agent. At room temperature it reacts readily with all elements (including otherwise "inert" elements like platinum and gold) except noble gases, nitrogen, oxygen and fluorine.[3]
Uses
Rocket propellant
Chlorine pentafluoride was once considered for use as an oxidizer for rockets. As a propellant, it has a higher maximum specific impulse than ClF3, but with the same difficulties in handling.[5] Due to the hazardous nature of chlorine pentafluoride and the large amounts of hydrogen fluoride in the exhaust, it has yet to be used in a large scale rocket propulsion system.
Safety
Chlorine pentafluoride is highly toxic. It is a strong irritant to skin, eyes and mucous membranes. It is very corrosive. Causes severe and painful skin, eye and respiratory tract burns. May cause lungs damage, toxic pneumonitis and permanent blindness. It is fatal if inhaled or absorbed through skin. Containers with chlorine pentafluoride may explode when heated. Ruptured cylinders may rocket violently. Chlorine pentafluoride causes other symptoms like nausea, vomiting and dyspnea.[1][9]
Chlorine pentafluoride reacts violently with water or moisture in the air and even water ice at −100 °C (−148 °F) (to produce corrosive hydrofluoric acid and toxic chlorine gas), nitric acid (even at −100° C), metals and organic materials. It is a strong oxidizer. It does not burn, but support burning. On catching fire or heated to decomposition, it emits corrosive and very toxic gases. Vapors from liquefied gas are initially heavier than air and spread along the floor. These are strong oxidizers and will react vigorously or explosively with many materials including fuels. May ignite flammable materials (wood, paper, oil, clothing, etc.).[9]
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.


