Calcium titanate
Chemical compound From Wikipedia, the free encyclopedia
Calcium titanate is an inorganic compound with the chemical formula CaTiO3. As a mineral, it is called perovskite, named after Russian mineralogist, Lev Perovski (1792–1856). It is a colourless, diamagnetic solid, although the mineral is often coloured owing to impurities.
 | |
| Names | |
|---|---|
| Other names
calcium titanium oxide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.031.795 |
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| CaTiO3 | |
| Molar mass | 135.943 g/mol |
| Appearance | white powder |
| Density | 4.1 g/cm3 |
| Melting point | 1,975 °C (3,587 °F; 2,248 K) |
| Boiling point | 3,000 °C (5,430 °F; 3,270 K) |
| insoluble | |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
>1200 mg/kg (oral, rat) |
| Thermochemistry | |
Std molar entropy (S⦵298) |
93.64 J/mol·K [1] |
Std enthalpy of formation (ΔfH⦵298) |
−1660.630 kJ/mol [1] |
Gibbs free energy (ΔfG⦵) |
−1575.256 kJ/mol [1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
CaTiO3 can be prepared by the combination of CaO and TiO2 at temperatures >1300 °C. Sol-gel processes has been used to make a more pure substance, as well as lowering the synthesis temperature. These compounds synthesized are more compressible due to the powders from the sol-gel process as well and bring it closer to its calculated density (~4.04 g/ml).[2]
Structure
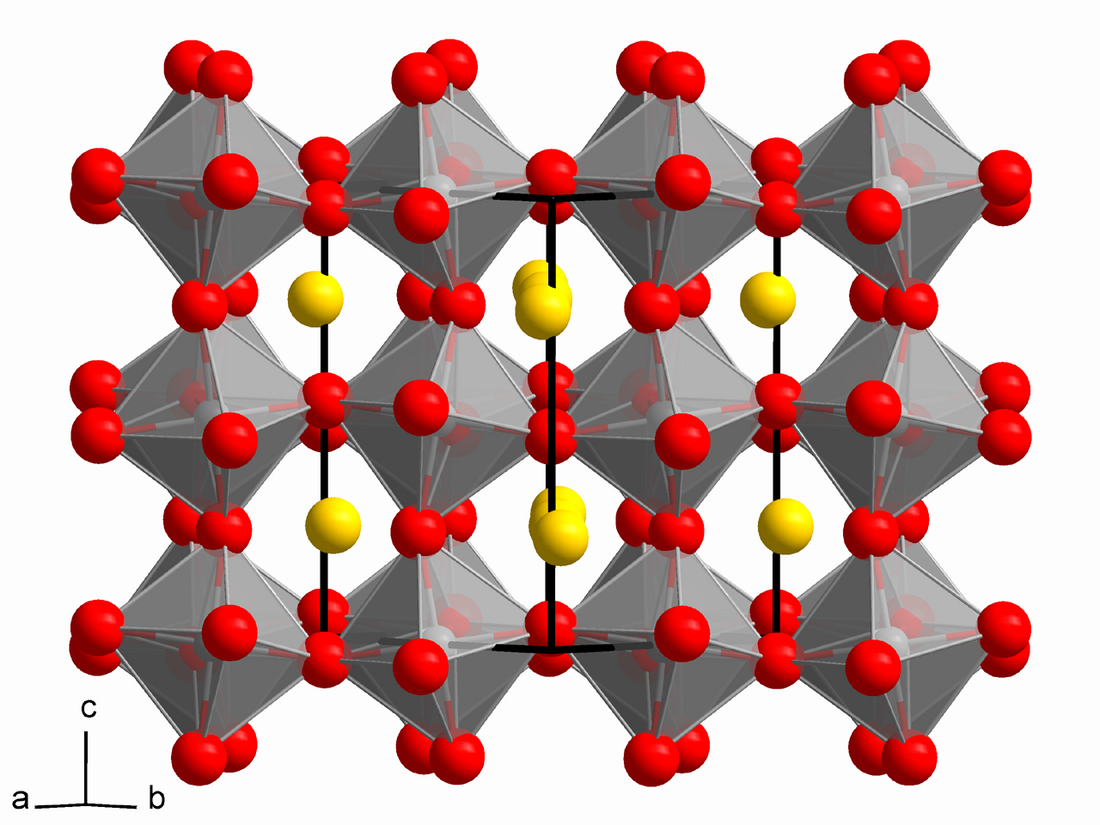
Calcium titanate is obtained as orthorhombic crystals, more specifically perovskite structure.[3] In this motif, the Ti(IV) centers are octahedral and the Ca2+ centers occupy a cage of 12 oxygen centres. Many useful materials adopt related structures, e.g. barium titanate or variations of the structure, e.g. yttrium barium copper oxide.[citation needed]
Applications
Calcium titanate has relatively little value except as one of the ores of titanium, together with several others. It is reduced to give titanium metal or ferrotitanium alloys.[4]
See also
References
External links
Wikiwand - on
Seamless Wikipedia browsing. On steroids.