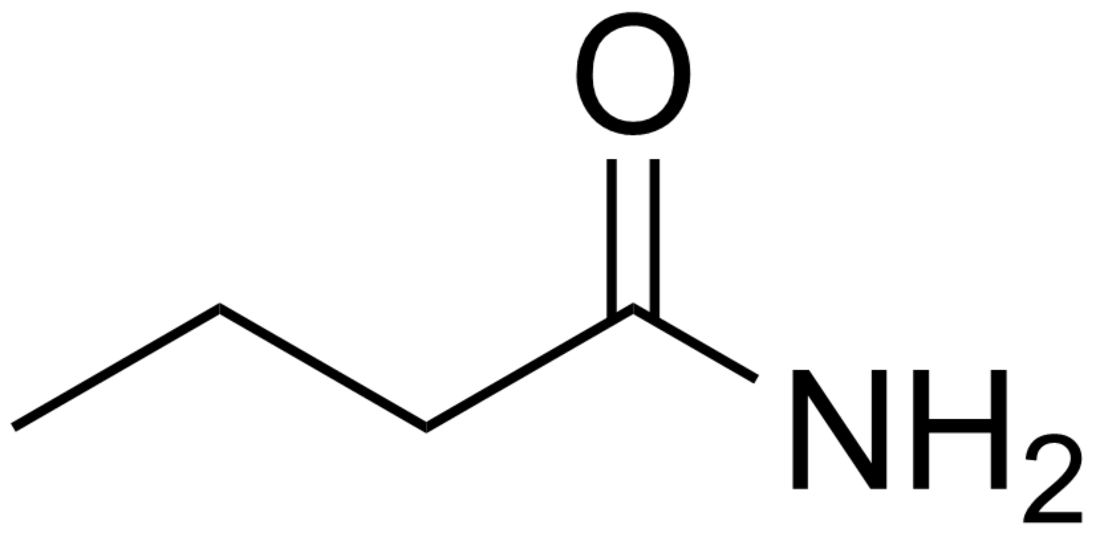
Butyramide
Chemical compound From Wikipedia, the free encyclopedia
Butyramide is the amide of butyric acid. It has the molecular formula C3H7CONH2. It is a white solid that is freely soluble in water and ethanol, but slightly soluble in diethyl ether. At room temperature, butyramide is a crystalline solid and in contrast to butyric acid, it is devoid of an unpleasant, rancid smell.
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Butanamide | |
| Other names
Butyramide n-Butanamide | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.007.980 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C4H9NO | |
| Molar mass | 87.122 g·mol−1 |
| Density | 1.03 g/cm3 |
| Melting point | 115 to 116 °C (239 to 241 °F; 388 to 389 K) |
| Boiling point | 216 °C (421 °F; 489 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Synthesis
Butyramide can be synthesized by:
- catalytic hydration of butyronitrile;
- reaction of butyryl chloride with ammonium salts;
- reduction of butyraldoxime.
Derivatives
Some of its derivatives have shown preliminary strong anticonvulsive activity and inhibitory action on histone deacetylases, which are crucial enzymes controlling the proliferative or differentiation status of most cells.
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.