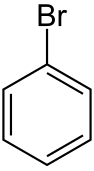
Bromobenzene
Chemical compound From Wikipedia, the free encyclopedia
Bromobenzene is an aryl bromide and the simplest of the bromobenzenes, consisting of a benzene ring substituted with one bromine atom. Its chemical formula is C6H5Br. It is a colourless liquid although older samples can appear yellow. It is a reagent in organic synthesis.
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Bromobenzene[1] | |||
| Other names
Phenyl Bromide Bromobenzol Monobromobenzene | |||
| Identifiers | |||
3D model (JSmol) |
|||
| 1236661 | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.295 | ||
| EC Number |
| ||
| KEGG | |||
PubChem CID |
|||
| RTECS number |
| ||
| UNII | |||
| UN number | 2514 | ||
CompTox Dashboard (EPA) |
|||
| |||
| |||
| Properties | |||
| C6H5Br | |||
| Molar mass | 157.010 g·mol−1 | ||
| Appearance | Colourless liquid | ||
| Odor | Pleasant aromatic odor | ||
| Density | 1.495 g cm−3, liquid | ||
| Melting point | −30.8 °C (−23.4 °F; 242.3 K) | ||
| Boiling point | 156 °C (313 °F; 429 K) | ||
| 0.041 g/100 mL | |||
| Solubility | soluble in diethyl ether, alcohol, CCl4 miscible in chloroform, benzene, petroleum ether | ||
| Vapor pressure | 4.18 mm Hg | ||
| −78.92·10−6 cm3/mol | |||
Refractive index (nD) |
1.5602 | ||
| Viscosity |
| ||
| Hazards | |||
| GHS labelling: | |||
   | |||
| Warning | |||
| H226, H315, H411 | |||
| P210, P233, P240, P241, P242, P243, P264, P273, P280, P302+P352, P303+P361+P353, P321, P332+P313, P362, P370+P378, P391, P403+P235, P501 | |||
| NFPA 704 (fire diamond) | |||
| Flash point | 51 °C (124 °F; 324 K) | ||
| 565 °C (1,049 °F; 838 K) | |||
| Related compounds | |||
Related halobenzenes |
Fluorobenzene Chlorobenzene Iodobenzene | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |||
Synthesis and reactions
Bromobenzene is prepared by the action of bromine on benzene in the presence of Lewis acid catalysts such as aluminium chloride or ferric bromide.[3]
Bromobenzene is used to introduce a phenyl group into other compounds. One method involves its conversion to the Grignard reagent, phenylmagnesium bromide. This reagent can be used, e.g. in the reaction with carbon dioxide to prepare benzoic acid.[4] Other methods involve palladium-catalyzed coupling reactions, such as the Suzuki reaction. Bromobenzene is used as a precursor in the manufacture of phencyclidine.
Toxicity
Animal tests indicate low toxicity.[5] Little is known about chronic effects.[6][7]
For liver toxicity, the 3,4-epoxide is a proposed intermediate.[8]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.