Benzyl iodide
Chemical compound From Wikipedia, the free encyclopedia
Chemical compound From Wikipedia, the free encyclopedia
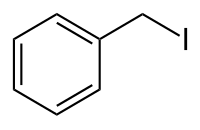
Benzyl iodide is an organic compound with the chemical formula C
7H
7I.[1][2] The compound consists of a benzene ring with an attached iodidemethyl group. The substance is an alkyl halide and is a constitutional isomer of the iodotoluenes.
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(Iodomethyl)benzene | |
| Other names
Fraissite, iodotoluol, α-iodotoluene, phenylmethyliodide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.009.659 |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C7H7I | |
| Molar mass | 218.037 g·mol−1 |
| Appearance | Low-melting crystals or colorless liquid |
| Melting point | 24.5 °C |
| Boiling point | 218 °C (424 °F; 491 K) |
| Insoluble | |
| Hazards | |
| GHS labelling: | |
  | |
| Warning | |
| Flash point | 86 °C (187 °F; 359 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Benzyl iodide can be obtained via the Finkelstein reaction from benzyl chloride and sodium iodide in acetone.

Benzyl iodide forms colorless to yellow needles, melting at 24.5 °C.[3] As a liquid, the compound has the high refractive index of 1.6334. Benzyl iodide is also a powerful lachrymator.[4][5]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.