Barr body
Form taken by the inactive X chromosome in a female somatic cell From Wikipedia, the free encyclopedia
A Barr body (named after discoverer Murray Barr)[1] or X-chromatin is an inactive X chromosome. In species with XY sex-determination (including humans), females typically have two X chromosomes,[2] and one is rendered inactive in a process called lyonization. Errors in chromosome separation can also result in male and female individuals with extra X chromosomes. The Lyon hypothesis states that in cells with multiple X chromosomes, all but one are inactivated early in embryonic development in mammals.[3][4] The X chromosomes that become inactivated are chosen randomly, except in marsupials and in some extra-embryonic tissues of some placental mammals, in which the X chromosome from the sperm is always deactivated.[5]

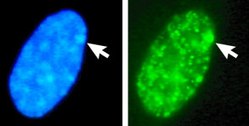
In humans with euploidy, a genotypical female (46, XX karyotype) has one Barr body per somatic cell nucleus, while a genotypical male (46, XY) has none. The Barr body can be seen in the interphase nucleus as a darkly staining small mass in contact with the nucleus membrane. Barr bodies can be seen in neutrophils at the rim of the nucleus.
In humans with more than one X chromosome, the number of Barr bodies visible at interphase is always one fewer than the total number of X chromosomes. For example, people with Klinefelter syndrome (47, XXY) have a single Barr body, and people with a 47, XXX karyotype have two Barr bodies.
History
Summarize
Perspective
1928 - Foundational Research
The discovery of the Barr body was based on the research of geneticist Emil Heitz studying the dynamics of moss chromatin during mitosis.[6] Heitz distinguished between heterochromatin and euchromatin, noting that certain regions of some chromosomes (and in some instances, entire chromosomes) retained their staining following mitosis.[6] This retained staining is indicative of condensed chromatin which he hypothesized, in the absence of mitosis, reflects silent regions of chromosomes (heterochromatin).[6]
1949 - Discovery of the Barr Body
Barr bodies were first discovered in 1949 by Canadian researcher Murray Barr and his undergraduate student Ewart Bertram.[7] While examining the neuronal cells of female cats, they observed a distinct, densely staining structure that was absent in male cells.[7] This structure was initially referred to as a "nucleolar satellite."[7] Although its significance was not immediately understood, the observation laid the foundation for subsequent research in cytogenetics.[7]
1955 - Development of the Buccal Smear Test
In 1955, Barr, in collaboration with K.L. Moore, developed the buccal smear test, a non-invasive method for collecting epithelial cells from the inner lining of the mouth.[8] This technique allowed the detection of Barr bodies in somatic cells and provided a simple tool for identifying chromosomal abnormalities, such as those seen in Turner syndrome and Klinefelter syndrome. The test became widely used in the mid-20th century and was among the earliest tools for determining chromosomal sex in clinical and research contexts.[8]
1959 - Identification of the Inactivated X Chromosome
In 1959, Japanese geneticist Susumu Ohno demonstrated that the previously identified "nucleolar satellite" was in fact the inactivated X chromosome in female somatic cells.[9] Using chromosomal staining techniques in animal models, such as rodents, he confirmed its identity and named it the "Barr body" in recognition of Barr's earlier discovery.[9] Ohno's work clarified that the Barr body was not merely a structural feature but represented the functional silencing of one X chromosome.[9]
1961 - Discovery of Lyonization
In 1961, British geneticist Mary Lyon proposed the concept of X chromosome inactivation, now known as Lyonization.[9] Her hypothesis suggested that in females, one of the two X chromosomes is randomly inactivated during early embryonic development to balance gene dosage.[10] This idea was based on her observations of genetic mosaicism in coat color patterns in mice.[10] Lyon's work provided a mechanistic explanation for the presence of the Barr body, linking it directly to the process of X inactivation.[9]
Mechanism
Summarize
Perspective
All individuals with two X chromosomes (such as the majority of human females) possesses only one Barr body per somatic cell, while all individuals with one X chromosome (such as most human males) have none.
The Barr body allows for equal expression of X chromosomes in the majority of human males and females.[11] If X inactivation did not occur, females (XX) would be expressing two X chromosomes, and males (XY) would only be expressing one. The disappearance of a Barr body in females (expressing both X chromosomes) can result in misregulation of heterochromatin. This misregulation leaves the potential of epigenetic instability and irregular gene expression.[12] Autosomal genes can be silenced when there is translocation of the X inactivation complex on the X chromosome to an autosome.[13]
Mammalian X-chromosome inactivation is initiated from the X inactivation centre or Xic, usually found near the centromere.[14] The centre contains twelve genes, seven of which code for proteins and five for untranslated RNAs. From the untranslated RNAs, only two are known to play an active role in the X inactivation process, Xist and Tsix.[14] The centre also appears to be important in chromosome counting: ensuring that random inactivation only takes place when two or more X-chromosomes are present. The provision of an extra artificial Xic in early embryogenesis can induce inactivation of the single X found in male cells.[14]
The roles of Xist and Tsix appear to be antagonistic. The loss of Tsix expression on the future inactive X chromosome results in an increase in levels of Xist around the Xic. Meanwhile, on the future active X Tsix levels are maintained; thus the levels of Xist remain low.[15] This shift allows Xist to begin coating the future inactive chromosome, spreading out from the Xic.[2] In non-random inactivation this choice appears to be fixed and current evidence suggests that the maternally inherited gene may be imprinted.[3] Variations in Xi frequency have been reported with age, pregnancy, the use of oral contraceptives, fluctuations in menstrual cycle and neoplasia.[16]
It is thought that this constitutes the mechanism of choice, and allows downstream processes to establish the compact state of the Barr body. These changes include histone modifications, such as histone H3 methylation (i.e. H3K27me3 by PRC2 which is recruited by Xist)[17] and histone H2A ubiquitination,[18] as well as direct modification of the DNA itself, via the methylation of CpG sites.[19] These changes help inactivate gene expression on the inactive X-chromosome and to bring about its compaction to form the Barr body. 3D reconstructions and microscopic analyses of the Barr body using chromosome painting have found that it has a smoother and rounder morphology than autosomes and the active X chromosome, though it is similar in size to the latter, suggesting its chromatin is only slightly more condensed.[6]
Role in cancer
Summarize
Perspective
The X chromosome encodes several tumour suppressor genes and oncogenes, thus incorrect dosage compensation may contribute to cancer development through their reactivation or silencing.[20] This could be achieved through poor epigenetic regulation of the Xi – it has been observed in several cancer types (medulloblastoma, glioblastoma, breast cancer, and acute myeloid leukemia) that the Xi accumulates more mutations than the autosomes.[20]
Reactivation of a Barr body is possible, and has been observed in breast and ovarian cancer cells.[21] One study showed that the frequency of Barr bodies in breast carcinoma was significantly lower than in healthy controls, indicating reactivation of previously inactivated X chromosomes.[21] In breast cancer cell lines, a loss of the repressive histone mark H3K27me3 was observed on the inactive X chromosome, disrupting its silenced state and leading to the expression of genes that are typically repressed.[22] This includes the bi-allelic expression of X-linked genes such as TBL1X and HDAC8, which may alter key pathways of transcriptional regulation, contributing to cancer pathogenesis.
It is more widely accepted that the loss of the Barr body in female cancers is the result of the duplication of the active X chromosome through mitotic error.[23] In any case, it is likely the abnormal over-expression of these X-linked genes that may contribute to tumour progression and cancer development.[22][23]
Uses
Summarize
Perspective
Barr Bodies in Ancient Samples: Observation and Relevance in Gender Identification of Extinct Species
Barr bodies are condensed, inactive X chromosomes found in the somatic cells of female mammals. Their detection in ancient samples provides a powerful tool for gender identification in extinct species, offering insights into population dynamics, biology, and evolution.
In forensic science, gender determination can be determined by analyzing dental pulp in Barr bodies.[24] Teeth are durable in the human body and is commonly used in forensics because of its characteristic of being less vulnerable to contamination by external DNA and its abundance in the body.[25] The presence of Barr bodies in dental pulp can be examined using histopathological and cytopathological techniques, where mean Barr body count is more in females than in male samples.[24] While the presence of Barr bodies is indicative of female sex, their absence is not sufficient to confirm male sex due to the possibility of chromosomal abnormalities or variations.[26]
Recent advancements in histological and genomic techniques have made it possible to observe Barr bodies in ancient remains, including fossilized bones and tissues:
- Histological Staining: Techniques like hematoxylin-eosin staining can highlight chromatin structures, including Barr bodies, in well-preserved osteocytes embedded within bone matrix.[27]
- Fluorescence Microscopy: Fluorescent dyes can differentiate X-chromosome condensation patterns, aiding in the visualization of Barr bodies.[27]
- Integration with Genomic Tools: Techniques such as PaleoHi-C enable the spatial reconstruction of chromosomal interactions, confirming the presence of inactivated X chromosomes in ancient samples.
In a notable example, Barr bodies were detected in osteocytes from ancient mammalian remains, demonstrating the potential of this approach for studying gender in extinct populations.[27]
Relevance in Gender Identification
- Population Studies:
- Identifying sex ratios in extinct species sheds light on social structures, reproductive strategies, and extinction dynamics.
- Reconstruction of Lifeways:
- Understanding the distribution of genders within ancient populations allows bioarchaeologists to analyze sex-based differences in diet, health, and activity patterns.[28]
- Preservation of Chromatin:
- The discovery of intact Barr bodies in fossils underscores the potential for studying chromosomal and epigenetic features in ancient samples
Limitations and Challenges
- Degradation of Samples: The fragmentation and chemical damage of ancient DNA and chromatin often hinder Barr body detection.
- Sample Availability: Successful detection depends on the preservation of osteocytes or other cells within the sample matrix.
- Replicability: Variability in preservation conditions can limit the reproducibility of results across samples.
Future Directions
Further research into the detection of Barr bodies may enhance our ability to:
- Identify sex in a broader range of extinct species.
- Study X-chromosome inactivation patterns across evolutionary timescales.
- Integrate histological and genomic methods to reconstruct detailed population dynamics.
Barr Body Analysis in Genetic Disorder Diagnosis
Barr bodies analysis is a rapid screening tool that can be used for preliminary identification of potential X chromosome aneuploidy. Results can be available within an hour, compared to karyotyping that typically takes 1-2 weeks.[29] Although Barr body analysis cannot provide a definitive diagnosis, as karyotyping is required for confirmation, diagnostic accuracy is high.[29] The conditions that can be identified include:
Turner Syndrome (45,X)
Turner syndrome is caused by the complete or partial absence of a second X chromosome in phenotypic females.[30] Approximately 50% of cases involve monosomy X, resulting in a 45,X karyotype that lacks a Barr body due to the presence of only one X chromosome.[30] In cases involving partial deletions or structural abnormalities of the second X chromosome, Barr bodies are typically also absent, as a fully functional second copy of the X chromosome is required for X inactivation and subsequent Barr body formation.[30]
Klinefelter Syndrome (47,XXY)
Klinefelter Syndrome is an aneuploidy in phenotypic males, characterized presence of two or more X chromosomes.[31] The most common karyotype is 47,XXY but other variations (48,XXXY, and 49,XXXXY) have been reported.[31] Unlike typical 46,XY males, individuals with Klinefelter syndrome undergo X chromosome inactivation, with the additional X chromosome forming a Barr body. In cases involving multiple extra X chromosomes, more than one Barr body may be observed.[31]
Triple X Syndrome (47,XXX)
Triple X Syndrome is an aneuploidy of the X chromosome in phenotypic females, resulting in an additional X chromosome.[32] Individuals with this condition typically have two Barr bodies per somatic cell, as two of the three X chromosomes undergo inactivation.[32]
See also
- X-inactivation
- Sex-determination system
- Nuclear sexing, a genetic sex determination technique
- Demethylation
- Acetylation
- Xist
- Tsix (gene)
References
Further reading
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
