Top Qs
Timeline
Chat
Perspective
Atrop-abyssomicin C
Chemical compound From Wikipedia, the free encyclopedia
Remove ads
Atrop-abyssomicin C is a polycyclic polyketide-type natural product that is the atropisomer of abyssomicin C. It is a spirotetronate that belongs to the class of tetronate antibiotics, which includes compounds such as tetronomycin, agglomerin, and chlorothricin.[1] In 2006, the Nicolaou group discovered atrop-abyssomicin C while working on the total synthesis of abyssomicin C.[2] Then in 2007, Süssmuth and co-workers isolated atrop-abyssomicin C from Verrucosispora maris AB-18-032, a marine actinomycete found in sediment of the Japanese sea. They found that atrop-abyssomicin C was the major metabolite produced by this strain, while abyssomicin C was a minor product. The molecule displays antibacterial activity by inhibiting the enzyme PabB (4-amino-4-deoxychorismate synthase), thereby depleting the biosynthesis of p-aminobenzoate.[3][4]
Remove ads
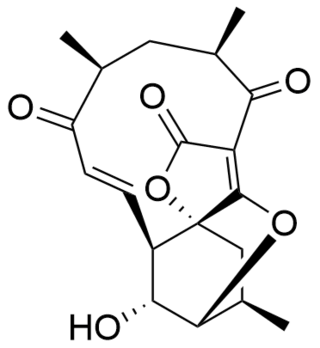
Structure

Atrop-abyssomicin C has a complex, yet intriguing structural topography. The compound contains an oxabicyclo[2.2.2]octane system fused to the tetronate moiety. The 11-membered macrocyclic ring carries an α,β-unsaturated ketone that was proposed to be the reactive center.[5] Despite being a strained macrocycle, there exist an atropisomer, abyssomicin C. The atropisomerism arise due to a structural deviation in the α,β-unsaturated ketone region of the molecule. The orientation of the carbonyl in atrop-abyssomicin C is cisoid, whereas the conformation in abyssomicin C is transoid.[6] The enone moiety of atrop-abyssomicin C has a higher degree of the conjugation, which makes it a more active Michael acceptor.[7]
Remove ads
Biosynthesis
Summarize
Perspective
The biosynthesis of atrop-abyssomicin C begins with the synthesis of a linear polyketide chain in a PKS I system that consist of one loading and six extension modules. The polyketide chain is made from five acetates, two propionates, and the glycolytic pathway metabolite. D-1,3-bisphosphoglycerate, the glycolytic metabolite, is transferred to AbyA3 (an acyl-carrier protein) by AbyA2 to generate the glyceryl-ACP. AbyA1 facilitates the attachment of the glyceryl-ACP to the polyketide chain and the detachment of the polyketide from the polyketide synthase to form intermediate 2.[7][8][9]

Based on the observation made for the biosynthesis of agglomerin, it has been proposed that AbyA4 acetylates intermediate 2 and AbyA5 catalyzes the elimination of acetic acid to form the exocyclic double bond in intermediate 4.[1] An intramolecular Diels-Alder was proposed to take place between the exocyclic olefin and the conjugated diene at the tail end of the polyketide to form the macrocyclic ring.[7] It has been reported that the previously unidentified Abycyc gene could code for an enzyme that carries out the Diels-Alder cycloaddition.[10] Following the Diels-Alder reaction, an epoxide ring is formed and then opened by the tetronate hydroxyl group to form atrop-abyssomicin C. It has been postulated that the AbyE monooxygenase catalyzes epoxide formation.[8]

Remove ads
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads