Ammonium hexachloroplatinate
Chemical compound From Wikipedia, the free encyclopedia
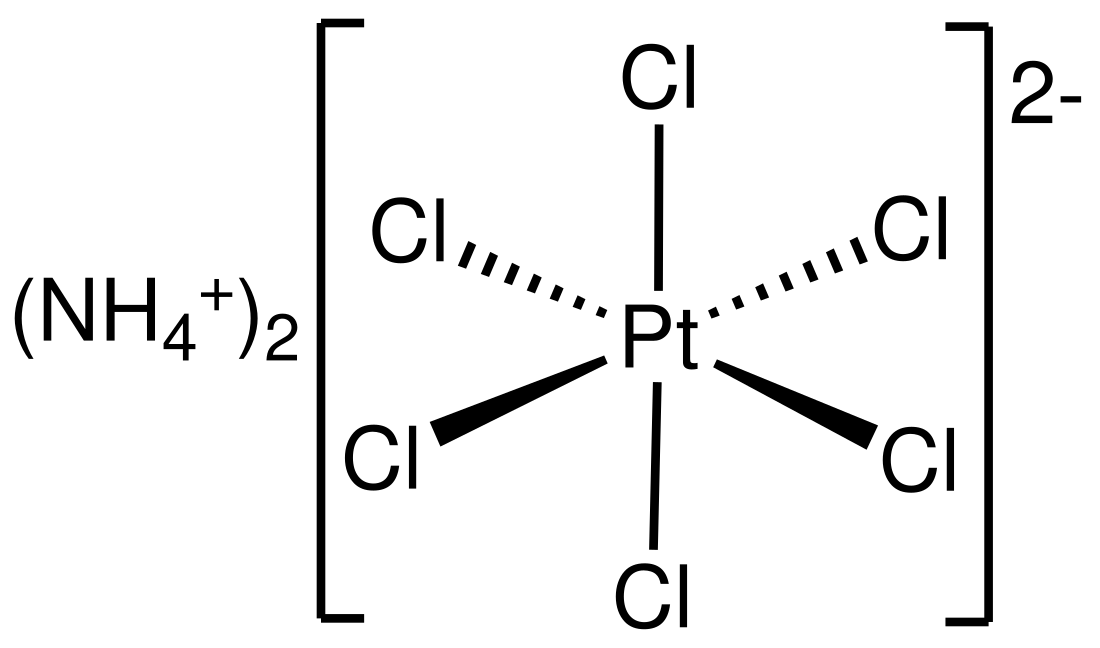
Ammonium hexachloroplatinate, also known as ammonium chloroplatinate, is the inorganic compound with the formula (NH4)2[PtCl6]. It is a rare example of a soluble platinum(IV) salt that is not hygroscopic. It forms intensely yellow solutions in water. In the presence of 1M NH4Cl, its solubility is only 0.0028 g/100 mL.
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Ammonium hexachloroplatinate(IV) | |
| Other names
ammonium chloroplatinate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.037.233 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| (NH4)2PtCl6 | |
| Molar mass | 443.87 g/mol |
| Appearance | yellow crystals |
| Odor | odorless |
| Density | 3.065 g/cm3 |
| Melting point | 380 °C (716 °F; 653 K) decomposes |
| 0.289 g/100ml (0 °C) 0.7 g/100ml (15 °C)[1] 0.499 g/100ml (20 °C) 3.36 g/100ml (100 °C) | |
| Hazards | |
| GHS labelling: | |
   | |
| Danger | |
| H290, H301, H317, H318, H334 | |
| P234, P261, P264, P270, P272, P280, P285, P301+P310, P302+P352, P304+P341, P305+P351+P338, P310, P321, P330, P333+P313, P342+P311, P363, P390, P404, P405, P501 | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
195 mg/kg rat |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation and structure
The compound consists of separate tetrahedral ammonium cations and octahedral [PtCl6]2− anions. It is usually generated as a fine yellow precipitate by treating a solution of hexachloroplatinic acid with a solution of an ammonium salt.[2] The complex is so poorly soluble that this step is employed in the isolation of platinum from ores and recycled residues.[3]
As analyzed by X-ray crystallography, the salt crystallizes in a cubic motif reminiscent of the fluorite structure. The [PtCl6]2− centers are octahedral. The NH4+ centers are hydrogen bonded to the chloride ligands.[4]
Uses and reactions
Ammonium hexachloroplatinate is used in platinum plating. Heating (NH4)2[PtCl6] under a stream of hydrogen at 200 °C produces platinum sponge. Treating this with chlorine gives H2[PtCl6].[2]
Ammonium hexachloroplatinate decomposes to yield platinum sponge when heated to high temperatures:[2][5]
- 3(NH4)2PtCl6 → 3Pt(s) + 2NH4Cl(g) + 16HCl(g) + 2N2(g)
Safety
Dust containing ammonium hexachloroplatinate can be highly allergenic. "Symptoms range from irritation of skin and mucous membranes to life-threatening attacks of asthma."[6]
Related compounds
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.