Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
Alloisoleucine is an amino acid with the formula CH3CH2CH(CH3)CH(NH2)CO2H. It exists as two enantiomers, of which the L derivative occurs naturally. L-Alloisoleucine occurs in healthy serum in only trace amounts, except for individuals suffering from maple syrup urine disease.[1]
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| 1721791 | |
| ChEBI |
|
| ChEMBL |
|
| ChemSpider | |
| DrugBank |
|
| ECHA InfoCard | 100.014.676 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Appearance | white solid |
| Melting point | 285 °C (545 °F; 558 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Together with valine, leucine, and isoleucine, alloisoleucine is classified as a branched-chain amino acid (BCAA). It is the rarest of the four.
L-Alloisoleucine is a diastereomer of the proteogenic amino acid L-isoleucine. The stereochemistry of the isobutyl group differs for L-alloisoleucine and L-isoleucine.
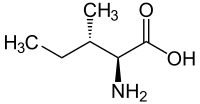  |
| l-isoleucine (2S,3S) and d-isoleucine (2R,3R) |
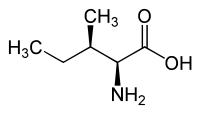  |
| l-alloisoleucine (2S,3R) and d-alloisoleucine (2R,3S) |
L-allo-isoleucine is a precursor to coronamic acid, which is a constituent of the phytotoxin coronatine, produced by Pseudomonas syringae.[2]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.