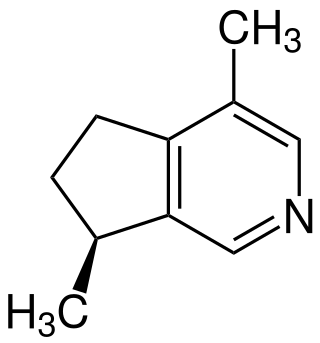
Actinidine
Chemical compound From Wikipedia, the free encyclopedia
Actinidine is an iridoid produced in nature by a wide variety of plants and animals. It was the first cyclopentanoid monoterpene alkaloid to be discovered.[2] It is one of several compounds that may be extracted from the valerian (Valeriana officinalis) root[3] and silver vine (Actinidia polygama), as well as several types of insects in the larval and imaginal stages.[4] Actinidine is a cat attractant, with effects like those of nepetalactone, the active compound found in catnip.[5]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
(7S)-4,7-Dimethyl-6,7-dihydro-5H-cyclopenta[c]pyridine | |
| Identifiers | |
3D model (JSmol) |
|
| 81308 | |
| ChEBI | |
| ChemSpider | |
| KEGG | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H13N | |
| Molar mass | 147.221 g·mol−1 |
| Melting point | < 25 °C (77 °F; 298 K) |
| Boiling point | 100 to 103 °C (212 to 217 °F; 373 to 376 K) at 9 mmHg[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Certain species of stick insects, including Megacrania batesii and Megacrania tsudai, possess a chemical defense mechanism which involves the secretion of an actinidine-containing substance from the prothoracic glands, when threatened by a predator.[6]
Biosynthesis
A potential biosynthesis of actinidine from L-citronellal is shown below.[7]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.