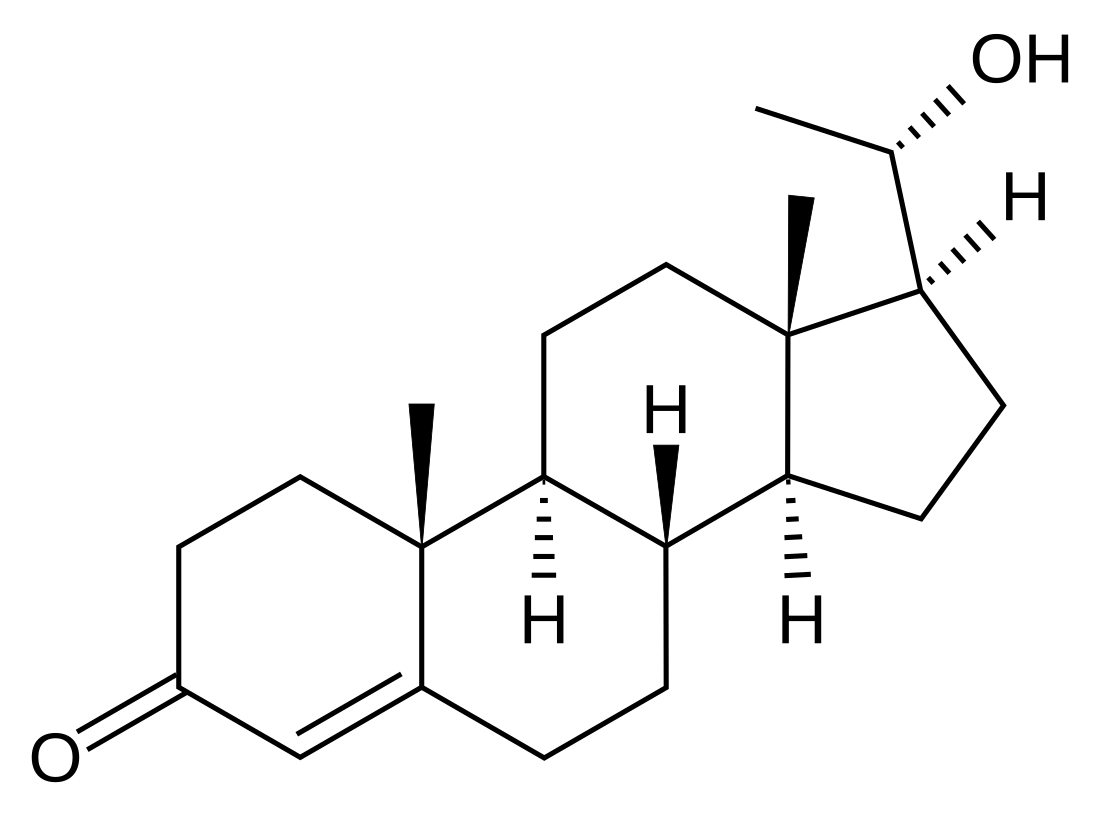
20α-Dihydroprogesterone
Chemical compound From Wikipedia, the free encyclopedia
20α-Dihydroprogesterone (20α-DHP), also known as 20α-hydroxyprogesterone (20α-OHP), is a naturally occurring, endogenous progestogen.[1][2][3] It is a metabolite of progesterone, formed by the 20α-hydroxysteroid dehydrogenases (20α-HSDs) AKR1C1, AKR1C2, and AKR1C3 and the 17β-hydroxysteroid dehydrogenase (17β-HSD) HSD17B1.[4][5] 20α-DHP can be transformed back into progesterone by 20α-HSDs and by the 17β-HSD HSD17B2.[6][7] HSD17B2 is expressed in the human endometrium and cervix among other tissues.[8][9][10][7] In animal studies, 20α-DHP has been found to be selectively taken up into and retained in target tissues such as the uterus, brain, and skeletal muscle.[6]
 | |
 | |
| Names | |
|---|---|
| IUPAC name
20α-Hydroxypregn-4-en-3-one | |
| Systematic IUPAC name
(1S,3aS,3bS,9aR,9bS,11aS)-1-[(1S)-1-Hydroxyethyl]-9a,11a-dimethyl-1,2,3,3a,3b,4,5,8,9,9a,9b,10,11,11a-tetradecahydro-7H-cyclopenta[a]phenanthren-7-one | |
| Other names
20α-DHP; 20α-Hydroxyprogesterone; 20α-OHP; Pregn-4-en-20α-ol-3-one | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
| MeSH | 20-alpha-Dihydroprogesterone |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C21H32O2 | |
| Molar mass | 316.478 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
20α-DHP has very low affinity for the progesterone receptor and is much less potent as a progestogen in comparison to progesterone, with about one-fifth of the relative progestogenic activity.[1][2][3][11][12][6][13] It has also been found to act as an aromatase inhibitor and to inhibit the production of estrogen in breast tissue in vitro.[14]
A single 200-mg oral dose of micronized progesterone has been found to result in peak levels of 20α-DHP of around 1 ng/mL after 2 hours.[15] In another study however, peak levels of 20α-DHP were around 10 ng/mL during therapy with 300 mg/day oral micronized progesterone.[16] 20α-DHP is formed from progesterone in the liver and in target tissues such as the endometrium.[16] It appears to be more slowly eliminated than progesterone.[16]
Levels of 5α-DHP have been quantified.[17]
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.