2-NBDG
Chemical compound From Wikipedia, the free encyclopedia
Chemical compound From Wikipedia, the free encyclopedia
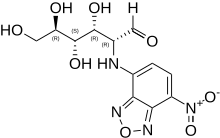
2-NBDG is a fluorescent tracer used for monitoring glucose uptake into living cells; it consists of a glucosamine molecule substituted with a 7-nitrobenzofurazan fluorophore at its amine group. It is widely referred to a fluorescent derivative of glucose,[1] and it is used in cell biology to visualize uptake of glucose by cells.[2] Cells that have taken up the compound fluoresce green.
 | |
| Names | |
|---|---|
| IUPAC name
2-(7-Nitro-2,1,3-benzoxadiazol-4-yl)-D-glucosamine | |
| Systematic IUPAC name
(2R,3R,4S,5R)-3,4,5,6-Tetrahydroxy-2-[(7-nitro-2,1,3-benzoxadiazol-4-yl)amino]hexanal | |
| Other names
2-NBD Glucose | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C12H14N4O8 | |
| Molar mass | 342.2646 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-NBDG is similar to radiolabeled glucose in that both can be used to detect glucose transport. Unlike radiolabeled glucose, 2-NBDG is compatible with fluorescence techniques such as a fluorescent microscopy, flow cytometry, and fluorimetry.[2]
The compound is taken up by a variety of mammalian, plant, and microbial cells[2][3][4] In mammalian cells, one transporter for 2-NBDG is GLUT2.,.[5] In bacterial cells, the predominant transporter is the mannose phosphotransferase system.[4] Cells that lack these or other compatible transporters do not take up 2-NBDG.[4][6] In T cells, 2-NBDG was transported by another, unidentified transporter.[7]
Like glucose, 2-NBDG is transported according to Michaelis–Menten kinetics. However, transport of 2-NBDG has a lower Vmax (maximum rate), and thus the rate of transport is generally slower than glucose.[4]
Once taken up, the compound is metabolized to a non-fluorescent derivative, as shown in Escherichia coli.[8] The identity and further metabolism of this non-fluorescent derivative has not been established.
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.