Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
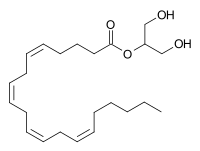
2-Arachidonoylglycerol (2-AG) is an endocannabinoid, an endogenous agonist of the CB1 receptor and the primary endogenous ligand for the CB2 receptor.[1][2] It is an ester formed from the omega-6 fatty acid arachidonic acid and glycerol. It is present at relatively high levels in the central nervous system, with cannabinoid neuromodulatory effects. It has been found in maternal bovine and human milk.[3] The chemical was first described in 1994–1995, although it had been discovered some time before that. The activities of phospholipase C (PLC) and diacylglycerol lipase (DAGL) mediate its formation.[4] 2-AG is synthesized from arachidonic acid-containing diacylglycerol (DAG).
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-O-[(5Z,8Z,11Z,14Z)-Icosa-5,8,11,14-tetraenoyl]glycerol | |
| Systematic IUPAC name
1,3-Dihydroxypropan-2-yl (5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoate | |
| Other names
2-AG, 2-arachidonoylglycerol | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C23H38O4 | |
| Molar mass | 378.3 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
2-AG, unlike anandamide (another endocannabinoid), is present at relatively high levels in the central nervous system; it is the most abundant molecular species of monoacylglycerol found in mouse and rat brain (~5–10 nmol/g tissue).[2][5] Detection of 2-AG in brain tissue is complicated by the relative ease of its isomerization to 1-AG during standard lipid extraction conditions. It has been found in maternal bovine as well as human milk.[6][7][8]
2-AG was discovered by Raphael Mechoulam and his student Shimon Ben-Shabat.[9] 2-AG was a known chemical compound but its occurrence in mammals and its affinity for the cannabinoid receptors were first described in 1994–1995. A research group at Teikyo University reported the affinity of 2-AG for the cannabinoid receptors in 1994–1995,[10][11] but the isolation of 2-AG in the canine gut was first reported in 1995 by the research group of Raphael Mechoulam at the Hebrew University of Jerusalem, which additionally characterized its pharmacological properties in vivo.[12] 2-Arachidonoylglycerol, next with Anandamide, was the second endocannabinoid discovered. The cannabinoid established the existence of a cannabinoid neuromodulatory system in the nervous system.[13]
Unlike anandamide, formation of 2-AG is calcium-dependent and is mediated by the activities of phospholipase C (PLC) and diacylglycerol lipase (DAGL).[2] 2-AG acts as a full agonist at the CB1 receptor.[14] At a concentration of 0.3 nM, 2-AG induces a rapid, transient increase in intracellular free calcium in NG108-15 neuroblastoma X glioma cells through a CB1 receptor-dependent mechanism.[2] 2-AG is hydrolyzed in vitro by monoacylglycerol lipase (MAGL), fatty acid amide hydrolase (FAAH), and the uncharacterized serine hydrolase enzymes ABHD2,[15] ABHD6 and ABHD12.[16] The exact contribution of each of these enzymes to the termination of 2-AG signaling in vivo is unknown, though it is estimated that MAGL is responsible for ~85% of this activity in the brain.[17] There have been identified transport proteins for 2-arachidonoylglycerol and anandamide. These include the heat shock proteins (Hsp70s) and fatty acid binding proteins (FABPs).[18][19]
2-Arachidonoylglycerol is synthesized from arachidonic acid-containing diacylglycerol (DAG), which is derived from the increase of inositol phospholipid metabolism by the action of diacylglycerol lipase. The molecule can also be formed from pathways like the hydrolysis derived (by diglyceride) from both phosphatidylcholine (PC) and phosphatidic acid (PAs) by the action of DAG lipase and the hydrolysis of arachidonic acid-containing lysophosphatidic acid by the action of a phosphatase.[20]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.