1-Tetralone
Chemical compound From Wikipedia, the free encyclopedia
1-Tetralone is a bicyclic aromatic hydrocarbon and a ketone. In terms of its structure, it can also be regarded as benzo-fused cyclohexanone. It is a colorless oil with a faint odor.[5] It is used as starting material for agricultural and pharmaceutical agents. The carbon skeleton of 1-tetralone is found in natural products such as Aristelegone A (4,7-dimethyl-6-methoxy-1-tetralone) from the family of Aristolochiaceae used in traditional Chinese medicine.[6]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
3,4-Dihydro-2H-naphthalen-1-one | |
| Other names
α-Tetralone; 1-Tetralone | |
| Identifiers | |
3D model (JSmol) |
|
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.007.692 |
| EC Number |
|
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C10H10O | |
| Molar mass | 146.189 g·mol−1 |
| Appearance | colorless liquid |
| Density | 1.099 g·cm−3 (25 °C)[1] |
| Melting point | 2–7 °C[1] |
| Boiling point | 255–257 °C[2] 113–116 °C (8 hPa)[1] |
| insoluble[3] | |
| Solubility | soluble in organic solvents |
| Vapor pressure | 2.7 Pa (20 °C)[3] |
Refractive index (nD) |
1.5672 |
| Hazards | |
| GHS labelling:[4] | |
 | |
| Warning | |
| H302 | |
| P264, P270, P301+P317, P330, P501 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
Summarize
Perspective
By oxidation of 1,2,3,4-tetrahydronaphthalene
As already described in 1933 by Heinrich Hock, 1,2,3,4-tetrahydronaphthalene tends to autoxidize and gradually forms the 1-hydroperoxide with atmospheric oxygen.[7] The heavy metal ion catalyzed air oxidation of 1,2,3,4-tetrahydronaphthalene with Cr3+[8] or Cu2+ in the liquid phase leads via the hydroperoxide to a mixture of the intermediate 1-tetralol and the final product 1-tetralone.[9]

The boiling points of the main component 1-tetralone (255-257 °C) and the minor component 1-tetralol (255 °C)[2] are virtually identical, the latter is therefore removed by a chemical reaction.[10]
By Friedel-Crafts reactions
The starting compound 4-phenylbutanoic acid is accessible from 3-benzoylpropanoic acid via catalytic hydrogenation, using a palladium contact catalyst.[5] 3-Benzoylpropanoic acid[11] itself can be obtained by a Haworth reaction (a variant of the Friedel-Crafts reaction) from benzene and succinic anhydride.
The intramolecular cyclization of 4-phenylbutanoic acid to 1-tetralone is catalyzed by polyphosphoric acid[5] and methanesulfonic acid.[12]

It has been described as a teaching experiment for chemistry lessons.[13] 4-Phenylbutanoic acid can also be quantitatively converted into 1-tetralone by heating in the presence of a strong Lewis acid catalyst such as bismuth(III)bis(trifluoromethanesulfonyl)amide[14] [Bi(NTf2)3], which is relatively easily accessible.[15]
The use of the acid chloride and tin(IV) chloride (SnCl4) allows significantly shorter reaction times than the Friedel-Crafts acylation with 4-phenylbutanoic acid.[10]

4-Phenylbutanoic acid chlorides with electron-donating groups can be cyclized to 1-tetralones under mild reaction conditions in yields greater than 90% using the strong hydrogen-bonding solvent hexafluoroisopropanol (HFIP).[16]
The AlCl3-catalyzed acylation of benzene with γ-butyrolactone produces 1-tetralone.[10]

Reactions
Summarize
Perspective
1-Tetralone can be reduced via a Birch reduction with lithium in liquid ammonia to 1,2,3,4-tetrahydronaphthalene.[17] The keto group can also be reduced to a secondary alcohol giving 1-tetralol, when a modified process is applied, using the addition of aqueous ammonium chloride solution after evaporation of the ammonia.[18]

With calcium in liquid ammonia, 1-tetralone is reduced to 1-tetralol at -33 °C in 81% yield.[19]
The methylene group in α-position to the keto group is particularly reactive and can be converted with formaldehyde (in the form of the trimeric trioxane) to 2-methylene-1-tetralone in the presence of the trifluoroacetic acid salt of N-methylaniline with yields up to 91% .

The 2-methylene ketone is stable at temperatures below -5 °C, but fully polymerizes at room temperature within 12 hours.[20]
In the Pfitzinger reaction of 1-tetralone with isatin, a compound called tetrofan (3,4-dihydro-1,2-benzacridine-5-carboxylic acid) is formed.

The reactivity of the α-methylene group is also exploited in the reaction of 1-tetralone with methanol at 270-290 °C, which produces via dehydrogenation and formation of the aromatic naphthalene ring system 2-methyl-1-naphthol in 66% yield.[21]

The oxime of 1-tetralone reacts with acetic anhydride leading to aromatization of the cycloalkanone ring. The resulting N-(1-naphthyl)acetamide[22] has biological properties akin to those of 2-(1-Naphthyl)acetic acid as a synthetic auxin.

The tertiary alcohol formed in the Grignard reaction of 1-tetralone with phenylmagnesium bromide reacts with acetic anhydride upon elimination of water to 1-phenyl-3,4-dihydronaphthalene, which is dehydrated with elemental sulfur in an overall yield of about 45% to 1-phenylnaphthalene.[23]

The ruthenium(II)-catalyzed arylation of 1-tetralone using phenyl boronic acid neopentyl glycol ester gives 8-phenyl-1-tetralone in up to 86% yield.[24]

With 5-aminotetrazole and an aromatic aldehyde, 1-tetralone reacts in a multi-component reaction under microwave irradiation to form a four-membered heterocyclic ring system.[25]

Applications
By far the most important application of 1-tetralone is in the synthesis of 1-naphthol by aromatization, e.g. upon contact with platinum catalysts at 200 to 450 °C.[26]
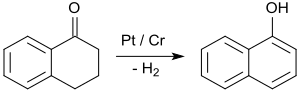
1-Naphthol is the starting material for the insecticides carbaryl and the beta-blockers propranolol.
Safety
Toxicological studies were dermally performed with rabbits, with an LD50 of 2192 mg·kg−1 body weight being observed.[1]
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
