Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
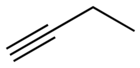
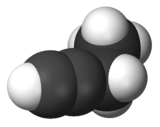
1-Butyne is an organic compound with the formula CH3CH2C≡CH. It is a terminal alkyne. The compound is a common terminal alkyne substrate in diverse studies of catalysis. It is a colorless combustible gas.[1]
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
But-1-yne | |
| Other names
Ethylacetylene Ethylethyne, UN 2452 | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.003.139 |
| EC Number |
|
PubChem CID |
|
| UNII | |
| UN number | 2452 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties[1] | |
| C4H6 | |
| Molar mass | 54.091 g/mol |
| Density | 0.6783 g cm−3[1] |
| Melting point | −125.7 °C (−194.3 °F; 147.5 K)[1] |
| Boiling point | 8.08 °C (46.54 °F; 281.23 K)[1] |
| Hazards | |
| GHS labelling: | |
 | |
| Danger | |
| H220 | |
| P210, P377, P381, P403 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1-Butyne participates in reactions typical for terminal alkynes, such as alkyne metathesis,[2] hydrogenation, condensation with formaldehyde. Based on its heat of combustion, it is slightly more stable than its isomer 2-butyne.[3]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.