Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
Tungsten hexabromide, also known as tungsten(VI) bromide, is a chemical compound of tungsten and bromine with the formula WBr6. It is an air-sensitive dark grey powder that decomposes above 200 °C to tungsten(V) bromide and bromine.[1][3]
 | |
| Names | |
|---|---|
Other names
| |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| WBr6 | |
| Molar mass | 663.264 g/mol |
| Appearance | Dark grey solid |
| Density | 5.32 g/cm3 |
| Melting point | 232 °C (450 °F; 505 K) (decomposition) |
| Hydrolysis | |
| Solubility | Soluble in ethanol, ether, carbon disulfide, and ammonia[1] |
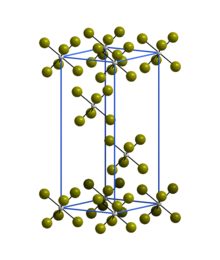
| Structure[2] | |
| Rhombohedral | |
| R3 | |
a = 6.39 Å, c = 17.53 Å | |
Lattice volume (V) |
620.8 Å3 |
Formula units (Z) |
3 |
| Related compounds | |
Other anions |
Tungsten hexafluoride Tungsten hexachloride |
Related compounds |
Tungsten(V) bromide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |

Tungsten hexabromide is mainly produced by the reaction of metallic tungsten and bromine at temperatures around 100 °C in a nitrogen atmosphere:[1][2]
Another method of producing this compound is by the reaction of tungsten hexacarbonyl and bromine at room temperature, releasing carbon monoxide.[4] It can also be produced by the metathesis reaction of boron tribromide and tungsten hexachloride.[5]
WBr6 is reduced with elemental antimony at elevated temperatrues, consecutively producing, WBr5, WBr4, W4Br10, W5Br12, then finally WBr2 at 350 °C. This reaction produces antimony tribromide as a side product.[4][6] Any of these bromides can be reverted to the hexabromide by oxidation with bromine at 160 °C.[7]
Tungsten hexabromide is hydrolyzed in water, producing tungsten pentoxide and releasing bromine.[1]
Tungsten(VI) oxytetrabromide is produced by the reaction of tungsten hexabromide and tungsten(VI) oxide:[7]
The trigonal crystal structure of WBr6 consists of isolated WBr6 octahedra and is isostructural with α-WCl6.[2]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.