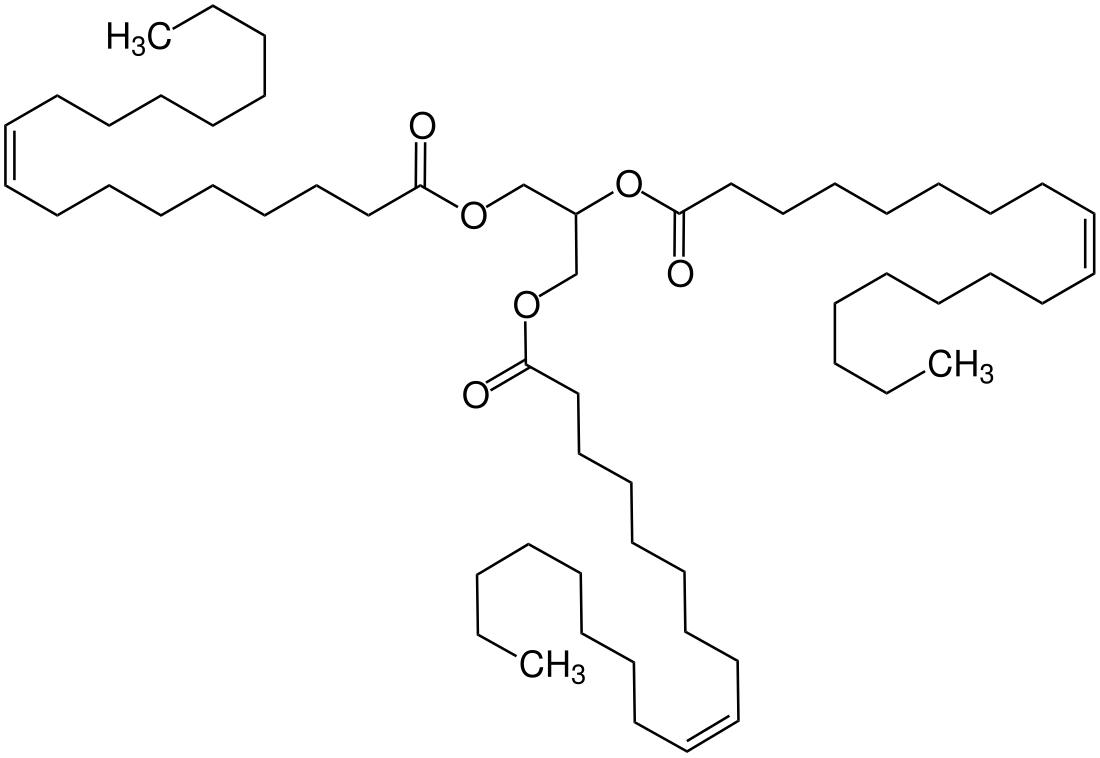
Triolein
Chemical compound From Wikipedia, the free encyclopedia
Triolein (glyceryl trioleate) is a symmetrical triglyceride derived from glycerol and three units of the unsaturated fatty acid oleic acid. Most triglycerides are unsymmetrical, being derived from mixtures of fatty acids. Triolein represents 4–30% of olive oil.[1]
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Propane-1,2,3-triyl tri[(9Z)-octadec-9-enoate] | |
| Other names
Glyceryl trioleate | |
| Identifiers | |
3D model (JSmol) |
|
| ChEBI | |
| ChemSpider | |
| ECHA InfoCard | 100.004.123 |
| MeSH | Triolein |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C57H104O6 | |
| Molar mass | 885.432 g/mol |
| Appearance | Colourless viscous liquid |
| Density | 0.9078 g/cm3 at 25 °C |
| Melting point | 5 °C; 41 °F; 278 K |
| Boiling point | 554.2 °C; 1,029.6 °F; 827.4 K |
| Solubility | Chloroform 0.1g/mL |
| Hazards | |
| Flash point | 302.6 °C (576.7 °F; 575.8 K) |
| Thermochemistry | |
Std enthalpy of formation (ΔfH⦵298) |
1.97·105 kJ/kmol |
Gibbs free energy (ΔfG⦵) |
−1.8·105 kJ/kmol |
Std enthalpy of combustion (ΔcH⦵298) |
8,389 kcal (35,100 kJ) /mole |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Triolein is also known as glyceryl trioleate and is one of the two components of Lorenzo's oil.[2]
The oxidation of triolein is according to the formula:
- C
57H
104O
6 + 80 O
2 → 57 CO
2 + 52 H
2O
This gives a respiratory quotient of 57/80 or 0.7125. The heat of combustion is 8,389 kcal (35,100 kJ) per mole or 9.474 kcal (39.64 kJ) per gram. Per mole of oxygen it is 104.9 kcal (439 kJ).
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.