Loading AI tools
Non-steroidal anti-inflammatory drug From Wikipedia, the free encyclopedia
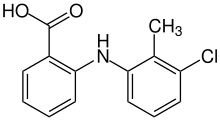
Tolfenamic acid (Clotam, Tufnil, TFA) is a member of the anthranilic acid derivatives (or fenamate) class of NSAID drugs.[2] Like other members of the class, it is a COX inhibitor and prevents formation of prostaglandins.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Clotam, Clotan, Tufnil, Migea |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.033.862 |
| Chemical and physical data | |
| Formula | C14H12ClNO2 |
| Molar mass | 261.71 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
It is used in the UK as a treatment for migraine.[4][5] It is generally not available in the US.[3] It is available in some Asian, Latin American and European countries as a generic drug for humans and for animals.[6]
TFA, like other non-steroidal anti-inflammatory drugs (NSAIDs), finds utility in the prevention and treatment of conditions associated with pain and inflammation.[7][8] However, despite its efficacy when administered intramuscularly, subcutaneously, or orally,[9] TFA-based drugs have not yet gained approval in the United States and some other countries due to the significant number of reported side effects.[10][11]
Nevertheless, TFA exhibits promise in medical practice, demonstrating the ability to inhibit the growth of cancer cells in the pancreas, sigmoid colon, and rectum.[12] Further research and development may unveil its potential for therapeutic applications in the future.
Tolfenamic acid, belonging to the pharmacological group of fenamates, possesses a chemical structure typical of anthranilic acid derivatives. In this structure, one of the hydrogen atoms of the nitro group is substituted by a benzene ring featuring a methyl group and a chlorine atom at the ortho- and meta- positions, respectively.[13]
Currently, nine forms of TFA have been identified, some of which are determined by conformational states.[14][15][16] These polymorphic forms exhibit variations in the spatial arrangement within the unit cell and in the values of the C-N(H)-C-C angle.[16] This diversity in solid forms makes TFA an attractive candidate for modification and utilization in medical applications.
It was discovered by scientists at Medica Pharmaceutical Company in Finland.[2]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.