Titanium nitrate is the inorganic compound with formula Ti(NO3)4. It is a colorless, diamagnetic solid that sublimes readily. It is an unusual example of a volatile binary transition metal nitrate. Ill defined species called titanium nitrate are produced upon dissolution of titanium or its oxides in nitric acid.
 | |
| Names | |
|---|---|
| Other names
titanium tetranitrate, tetranitratotitanium | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.222.601 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| Ti(NO3)4 | |
| Molar mass | 295.8866 g/mol |
| Appearance | white volatile solid |
| Density | 2.192[3] |
| Melting point | 58[4] °C (136 °F; 331 K) |
| Boiling point | decompose |
| Reacts[5] | |
| Structure[6] | |
| monoclinic | |
| P21/C | |
a = 7.80, b = 13.57, c = 10.34 Å α = 90°, β = 125·0°, γ = 90° | |
Lattice volume (V) |
896.52 Å3 |
Formula units (Z) |
4 |
| 8 | |
| flattened tetrahedral | |
| Related compounds | |
Related compounds |
hafnium nitrate, zirconium nitrate, titanium phosphate, titanium perchlorate |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation
Similarly to its original method,[7][8] Ti(NO3)4 is prepared by the nitration of titanium tetrachloride using dinitrogen pentoxide[9] or chlorine nitrate:[10]
- TiCl4 + 4 N2O5 → Ti(NO3)4 + 4 ClNO2
Hydrated titanium nitrate, the nitrate salt of the aquo complex [Ti(H2O)6]3+, is produced upon dissolution of titanium compounds in nitric acid.[11]
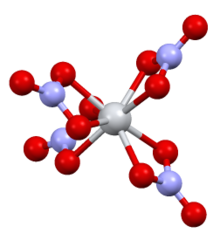
Structure
The complex has D2d symmetry, with four bidentate nitrate ligands. The N-O distances are 1·29 Å and 1·185 Å (noncoordinated).[6]
Physical properties
In the infrared spectrum, it absorbs strongly at 1635 cm−1, assigned to a N-O vibrational mode.[12]
It is soluble in nonpolar solvents silicon tetrachloride and carbon tetrachloride.[13][8]
Reactions
Titanium nitrate is hygroscopic, converting to ill-defined hydrates.[14] The anhydrous material is highly reactive, even toward hydrocarbons.[14] Titanium nitrate also reacts with n-dodecane,[15] p-dichlorobenzene, anisole, biphenyl,[15][16]
It decomposes thermally to titanium dioxide.[17]
References
Other reading
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.
