Recrystallization is a method used to purify chemicals by dissolving a mixture of a compound and its impurities, in an appropriate solvent, prior to heating the solution.[1] Following the dissolution of crude product, the mixture will passively cool, yielding a crystallized compound and its impurities as separate entities. The newly formed crystals can then be subjected to x-ray anaylsis for purity assessment.[2]
This article needs additional citations for verification. (October 2009) |
Methods
Summarize
Perspective
Single-solvent recrystallization
The solvent utilized in single-solvent recrystallization must dissolve the crude reaction mixture only when it is heated to reflux.[3] The heated solution is then passively cooled, yielding a crystallized product absent of impurities.[3] The solid crystals are then collected utilizing a filtration apparatus and the filtrate is discarded.[4] Product purity can then be assessed via NMR spectroscopy.[5]

Multi-solvent recrystallization
Multi-solvent recrystallization relies on the crude product being soluble in one solvent, when it is heated to reflux, while being insoluble in a secondary solvent, regardless of the solvent's temperature.[6] The volume ratio between the first and second solvent is critical. A higher ratio of first to second solvent will lead to permanent dissolution of the desired product, while a low ratio will lead to minimal pure crystal recovery. The terms first and second are in reference to crude product soluble and crude product insoluble solvents respectively. Typically, the second solvent, following the dissolution of the impure solid in the first solvent, is added slowly until the desired product begins to crystallize from solution. The solution is then cooled to further induce recrystallization.[6]

Hot filtration recrystallization
Hot filtration recrystallization can be used to separate a pure compound from both impurities and some insoluble matter, which may be anything from a third party impurity to fragments of broken glass.[7] The technique makes use of the single solvent system, outlined above, by dissolving a crude reaction mixture, in a minimum amount of hot solvent, before gravity filtering the saturated solution to remove insoluble matter. The saturated solution will then passively cool, yielding pure crystals.[7]

X-ray analysis
Summarize
Perspective
Recrystallized products are often subject to X-ray crystallography for purity assessment.[8] The technique requires crystallized products to be singular, and absent of clumps.[8] Several approaches to this phenomenon are listed below.
- Slow evaporation of a single solvent - typically the compound is dissolved in a suitable solvent and the solvent is allowed to slowly evaporate. Once the solution is saturated crystals can be formed.
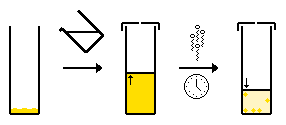
- Slow evaporation of a multi-solvent system - the same as above, however as the solvent composition changes due to evaporation of the more volatile solvent. The compound is more soluble in the volatile solvent, and so the compound becomes increasingly insoluble in solution and crystallizes.

- Slow diffusion - similar to the above. However, a second solvent is allowed to evaporate from one container into a container holding the compound solution (gas diffusion). As the solvent composition changes due to an increase in the solvent that has gas diffused into the solution, the compound becomes increasingly insoluble in the solution and crystallizes.
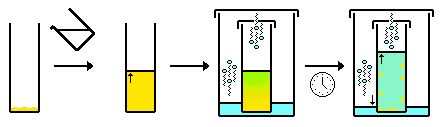
- Interface/slow mixing (often performed in an NMR tube). Similar to the above, but instead of one solvent gas-diffusing into another, the two solvents mix (diffuse) by liquid-liquid diffusion. Typically a second solvent is "layered" carefully on top of the solution containing the compound. Over time the two solution mix. As the solvent composition changes due to diffusion, the compound becomes increasingly insoluble in solution and crystallizes, usually at the interface. Additionally, it is better to use a denser solvent as the lower layer, and/or a hotter solvent as the upper layer because this results in the slower mixing of the solvents.

- Specialized equipment can be used in the shape of an "H" to perform the above, where one of the vertical lines of the "H" is a tube containing a solution of the compound, and the other vertical line of the "H" is a tube containing a solvent which the compound is not soluble in, and the horizontal line of the "H" is a tube which joins the two vertical tubes, which also has a fine glass sinter that restricts the mixing of the two solvents.

- Once single perfect crystals have been obtained, it is recommended that the crystals are kept in a sealed vessel with some of the liquid of crystallization to prevent the crystal from 'drying out'. Single perfect crystals may contain solvent of crystallization in the crystal lattice. Loss of this internal solvent from the crystals can result in the crystal lattice breaking down, and the crystals turning to powder.
See also
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.