Loading AI tools
Oxetene is an unsaturated heterocycle. The compound is unstable and has been synthesized.[1] Compared to oxetane, the saturated compound, oxetene is destabilized because the double bond increases the ring strain. Synthesis of some substituted derivatives has been reported.[2][3]
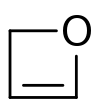 | |
| Names | |
|---|---|
| Preferred IUPAC name
2H-Oxete | |
| Systematic IUPAC name
1-Oxacyclobut-2-ene | |
| Identifiers | |
3D model (JSmol) |
|
| 4652799 | |
| ChEBI | |
| ChemSpider | |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C3H4O | |
| Molar mass | 56.06326 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Oxetene can be synthesised by the photochemical cyclization of acrolein:[4]

Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.