Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
Osaterone acetate, sold under the brand name Ypozane, is a medication which is used in veterinary medicine for the treatment of enlarged prostate in dogs.[3][5][6] It is given by mouth.[3]
 | |
| Clinical data | |
|---|---|
| Trade names | Ypozane |
| Other names | TZP-4238; Gestoxarone acetate; 2-Oxachloromadinone acetate; 17α-Acetoxy-6-chloro-2-oxa-6-dehydroprogesterone; 17α-Acetoxy-6-chloro-2-oxapregna-4,6-diene-3,20-dione, Osaterone acetate (JAN JP) |
| Routes of administration | By mouth (tablets) |
| Drug class | Steroidal antiandrogen; Progestogen; Progestin; Progestogen ester |
| ATCvet code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | Osaterone acetate: 90% 15β-Hydroxyosaterone acetate: 80%[3] (Both mainly to albumin)[3] |
| Metabolism | Liver[3] |
| Metabolites | 15β-Hydroxyosaterone acetate[3] |
| Elimination half-life | Dogs: 80 hours to 197 ± 109 hours[3][4] |
| Excretion | Bile: 60%[3] Urine: 25%[3] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.750 |
| Chemical and physical data | |
| Formula | C22H27ClO5 |
| Molar mass | 406.90 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Osaterone acetate is an antiandrogen, and hence is an antagonist of the androgen receptor, the biological target of androgens like testosterone and dihydrotestosterone.[3] It is also a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[3]
Osaterone acetate was introduced for veterinary use in 2007.[1][3][7][8]
Osaterone acetate is used in veterinary medicine for the treatment of benign prostatic hyperplasia (BPH) in dogs.[3][5][6] It has been found to produce remission of clinical symptoms of BPH in 83% of dogs for six months after a single one-week course of treatment,[9] and can be used long-term.[6]
Osaterone acetate comes in the form of 1.875 mg, 3.75 mg, 7.5 mg, and 15 mg oral tablets for veterinary use.[3]
Side effects of osaterone acetate include diminished sperm quality (for up to 6 weeks post-treatment), transient elevation of liver enzymes (caution should be observed with known liver disease), vomiting, diarrhea, polyuria/polydipsia, lethargy, and hyperplasia of the mammary glands.[10] It can also decrease cortisol levels, interfere with adrenocorticotropic hormone response, induce or exacerbate adrenal insufficiency, and exacerbate diabetes mellitus.[11][10]
Osaterone acetate is a steroidal antiandrogen, progestin, and antigonadotropin.[3] It has virtually no estrogenic or androgenic activity.[5] Its side-effect profile indicates that it possesses clinically relevant glucocorticoid activity.[11][10] An active metabolite of osaterone acetate, 15β-hydroxyosaterone acetate, has potent antiandrogenic activity similarly to osaterone acetate.[3] Osaterone acetate treats BPH in dogs by reducing the actions of androgens in the prostate gland.[3]
The major active metabolite of osaterone acetate is 15β-hydroxyosaterone acetate.[3] Osaterone acetate has a long biological half-life of 80 hours to 197 ± 109 hours in dogs.[3][4]
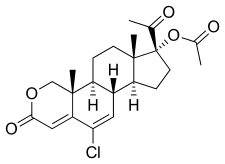
Osaterone acetate, also known as 2-oxachloromadinone acetate, as well as 17α-acetoxy-6-chloro-2-oxa-6-dehydroprogesterone or 17α-acetoxy-6-chloro-2-oxapregna-4,6-diene-3,20-dione, is a synthetic pregnane steroid and a derivative of progesterone and 17α-hydroxyprogesterone.[8] It is a derivative of the less potent chlormadinone acetate.[5] The medication is the C17α acetate ester of osaterone.[8]
Osaterone acetate was approved for veterinary use in the European Union under the brand name Ypozane in 2007.[1][3][7][8]
Osaterone acetate is the generic name of the drug.[1][8] Osaterone is the INN of the deacetylated parent compound.[8]
Osaterone acetate is marketed under the brand name Ypozane by Virbac throughout the European Union.[1][8]
Osaterone acetate was also investigated in Japan in the treatment of prostate cancer and BPH in humans but was ultimately never marketed for such purposes.[5][12]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.