Molybdenum dichloride dioxide
Chemical compound From Wikipedia, the free encyclopedia
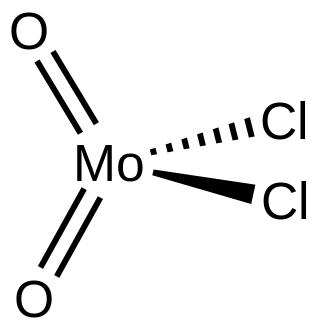
Molybdenum dichloride dioxide is the inorganic compound with the formula MoO2Cl2. It is a yellow diamagnetic solid that is used as a precursor to other molybdenum compounds. Molybdenum dichloride dioxide is one of several oxychlorides of molybdenum.
 | |
| Identifiers | |
|---|---|
3D model (JSmol) |
|
| ChemSpider | |
| ECHA InfoCard | 100.157.480 |
| EC Number |
|
PubChem CID |
|
| |
| |
| Properties | |
| Cl2MoO2 | |
| Molar mass | 198.85 g·mol−1 |
| Appearance | yellow or cream solid |
| Melting point | 175 °C (347 °F; 448 K) |
| Hazards | |
| GHS labelling: | |
 | |
| Danger | |
| H314 | |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Structure
Gaseous molybdenum dichloride dioxide is a monomer,[1] but upon condensation, it polymerizes to give a coordination polymer of uncertain structure.
Preparation
The compound is most easily prepared by treatment molybdenum trioxide with concentrated hydrochloric acid:[2]
- MoO3 + 2 HCl → MoO2Cl2 + H2O
MoO2Cl2 can also be prepared from MoOCl4:[3]
- MoOCl4 + O(Si(CH3)3)2 → MoO2Cl2 + 2 ClSi(CH3)3
It is also prepared by chlorination of molybdenum dioxide:[4]
- MoO2 + Cl2 → MoO2Cl2
It is also prepared by chlorination of molybdenum trixoide:[5]
- MoO3 + Cl2 → MoO2Cl2
Reactions
Many bisadducts are known of the type MoO2Cl2(ether)2. These octahedral molecular complexes are soluble in organic solvents.
With bulky anilines, it converts to the diimido complex MoCl2(NAr)2(dimethoxyethane). This complex is the precursor to the Schrock carbenes of the type Mo(OR)2(NAr)(CH-t-Bu).[4]
Related compounds
Molybdenum difluoride dioxide exists as a sublimable white solid, in contrast to the dichloride.
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.