Lithium cyclopentadienide
Chemical compound From Wikipedia, the free encyclopedia
Lithium cyclopentadienide is an organolithium compound with the formula C5H5Li. The compound is often abbreviated as LiCp, where Cp− is the cyclopentadienide anion. Lithium cyclopentadienide is a colorless solid, although samples often are pink owing to traces of oxidized impurities.
 | |
| Names | |
|---|---|
| Other names
lithium cyclopentadienylide, cyclopentadienyllithium, LiCp | |
| Identifiers | |
| ECHA InfoCard | 100.156.001 |
PubChem CID |
|
CompTox Dashboard (EPA) |
|
| Properties | |
| C5H5Li | |
| Molar mass | 72.04 g·mol−1 |
| Appearance | colorless solid |
| Density | 1.064 g/cm3 |
| decomposition | |
| Solubility | THF, dimethoxyethane |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Preparation, structure and reactions
Lithium cyclopentadienide is commercially available as a solution in tetrahydrofuran. It is prepared by treating cyclopentadiene with butyllithium:[1]
- C5H6 + LiC4H9 → LiC5H5 + C4H10
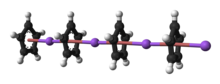
Because lithium cyclopentadienide is usually handled as a solution, the solvent-free solid is rarely encountered. According to X-ray crystallography, LiCp is a "polydecker" sandwich complex, consisting of an infinite chain of alternating Li+ centers sandwiched between μ-η5:η5-C5H5 ligands.[2] In the presence of amines or ethers, LiCp gives adducts, e.g. (η5-Cp)Li(TMEDA).[1] LiCp is a common reagent for the preparation of cyclopentadienyl complexes.
See also
References
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.
