Top Qs
Timeline
Chat
Perspective
Isotopes of neon
From Wikipedia, the free encyclopedia
Remove ads
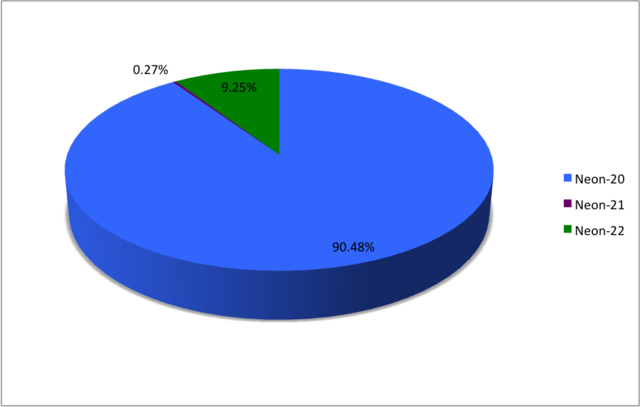
Neon (10Ne) naturally occurs as three stable isotopes: 20
Ne, 21
Ne, and 22
Ne. Their stated natural abundances are those measured in air.
In addition, 17 artificial radioisotopes have been discovered, ranging from 15
Ne to 34
Ne, all short-lived: the most stable is 24
Ne with a half-life of 3.38 minutes. All others are under a minute, and most under a second. The isotopes lighter than the stable ones usually decay to fluorine or oxygen, while heavier ones decay to sodium.
Remove ads
List of isotopes
Summarize
Perspective
- mNe – Excited nuclear isomer.
- ( ) – Uncertainty (1σ) is given in concise form in parentheses after the corresponding last digits.
- # – Atomic mass marked #: value and uncertainty derived not from purely experimental data, but at least partly from trends from the Mass Surface (TMS).
- Modes of decay:
n: Neutron emission p: Proton emission - Bold symbol as daughter – Daughter product is stable.
- ( ) spin value – Indicates spin with weak assignment arguments.
Remove ads
See also
Daughter products other than neon
References
Wikiwand - on
Seamless Wikipedia browsing. On steroids.
Remove ads
