Freshwater acidification
Acidification of freshwater by rain From Wikipedia, the free encyclopedia
Acidification of freshwater by rain From Wikipedia, the free encyclopedia
Freshwater acidification occurs when acidic inputs enter a body of fresh water through the weathering of rocks, invasion of acidifying gas (e.g. carbon dioxide), or by the reduction of acid anions, like sulfate and nitrate within a lake, pond, or reservoir.[1] Freshwater acidification is primarily caused by sulfur oxides (SOx) and nitrogen oxides (NOx) entering the water from atmospheric depositions and soil leaching.[1] Carbonic acid and dissolved carbon dioxide can also enter freshwaters, in a similar manner associated with runoff, through carbon dioxide-rich soils.[1] Runoff that contains these compounds may incorporate acidifying hydrogen ions and inorganic aluminum, which can be toxic to marine organisms.[1] Acid rain also contributes to freshwater acidification.[2] A well-documented case of freshwater acidification in the Adirondack Lakes, New York, emerged in the 1970s, driven by acid rain from industrial sulfur dioxide (SO₂) and nitrogen oxide (NOₓ) emissions.[3]
This article needs attention from an expert in Limnology and Oceanography. The specific problem is: Major revisions and clarifications needed. (March 2022) |
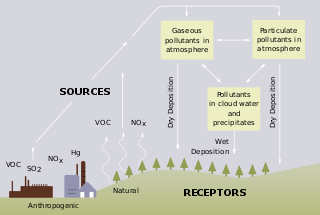
Atmospheric CO2 affects freshwater acidity.[4] Microbial activity breaks down of organic matter releases organic acids such as humic and fulvic acids. These acids accumulate in water bodies, especially those surrounded by forests and wetlands.[5] Peatlands and wetlands often produce acidic waters because of the high levels of organic matter decomposition.[6] This creates naturally acidic conditions, which are common in boreal and subarctic regions.
Volcanic activity can release sulfur dioxide (SO₂) and other acidic oxides into the atmosphere.[7] In air, sulfur dioxide converts to sulfuric acid:[8]

Human activities can significantly accelerate freshwater acidification. In addition to carbon dioxide, the combustion of fossil fuels sulfur dioxide (SO₂) and nitrogen oxides (NOₓ). These gases react with water and air to form sulfuric acid (H₂SO₄) and nitric acid (HNO₃).[7][9][10] This process is particularly harmful in areas where the natural buffering capacity of the water is low, as these ecosystems are less able to neutralize the added acidity.
Mining can significantly contribute to freshwater acidification through the process of acid mine drainage. When sulfide minerals such as pyrite (FeS₂) are exposed to air and water during mining operations, they oxidize to form sulfuric acid.[11]

The buffering capacity of ecosystems helps them resist changes in pH. When this is lacking, freshwater reservoirs become acidified. For example, the Atlantic region of Canada has the lowest acid deposition rates in Eastern North America, yet it has the most acidic waters on the continent due to the low buffering capacity of the regional bedrock and the addition of natural organic acids produced from close by wetlands. In most of the Atlantic region, granite and shale bedrock are found, which contain very little buffering material. Soil formed from low-buffering materials and the waters that drain from them are, therefore, susceptible to acidification, even under low acid deposition.[12]
Soil that undergoes acidification can negatively impact agriculture.[13] Some species are able to withstand low pH levels in their environment. For example, frogs and perches can withstand a pH level of 4.[14] This allows these species to be unaffected by the acid deposition in their aquatic environment, allowing them to survive in these conditions.[14] However, most aquatic species, such as clams and snails, are unable to withstand low pH levels which negatively impacts their growth and survival. The high acidic levels deteriorate their thick shells decreasing their protection from predators.[14]
Acidification of freshwater ecosystems can decrease native biodiversity and can alter ecosystem structure and function entirely.[8] Macro-invertebrates and large vertebrates exhibit higher mortality and lower reproductive rates under acidified conditions. Conversely, algae thrive in acidified environments, and may quickly dominate these habitats, outcompeting other species. In particular, it is common to see an increase in the abundance of the sphagnum. Sphagnum has a high capacity to exchange H+ for basic cations within freshwater. The thick layer of sphagnum restricts the exchange between surface water and sediment, further contributing to reduction in nutrient cycling in the ecosystem.[8] Aquatic biomonitoring can be used to examine the health of aquatic ecosystems.
Agricultural runoff is a major source of nitrogen and phosphorus, which contribute to freshwater acidification. Implementing best management practices (BMPs) in agriculture, such as reducing the use of chemical fertilizers, improving manure management, and adopting precision agriculture techniques, can significantly reduce nutrient runoff into water bodies.[15] Establishing riparian buffer zones—strips of vegetation planted along water bodies—can also help to filter pollutants from agricultural fields before they reach freshwater systems.[16] These measures not only reduce acidification but also mitigate eutrophication and improve overall water quality.
Wetlands and peatlands serve as buffers for freshwater systems by absorbing pollutants regulating water flow.[17] Wetland restoration projects have been shown to increase the resilience of freshwater systems to acidification and other environmental stressors.[18]
Liming, where calcium carbonate (CaCO3) is added to these system, increase pH levels.[19]
Regulation of anthropogenic emissions, specifically SOx and NOx, can lead to large decreases of acid rain and acidic bodies of water.[20] For example, the Canada-United States Air Quality Agreement has greatly minimized acid rain and ozone levels by 78% in Canada and 92% in the United States, as of 2020.[21] Moreover, investing in scientists to monitor and collect data is essential to create a model used to establish successful policies.[22] For instance, a protocol can be implemented to mitigate the issue.[22] Also, governments could invest funds to subsidize companies to decrease their pollution and incentivize them to use innovative methods of production, to lower both greenhouse gas emissions and the amount of acidic substances created. Furthermore, government institutions across the globe can connect on the issue of acidification and work together to find a feasible solution through international agreements.[13] Some successful government implementations include the Acid Rain Program[23] established in the United States in 1995, and the most recent Gothenburg Protocol, established by the United Nations Economic Commission for Europe (UNECE) to reduce acidification.[24]
One of the most well-documented cases of freshwater acidification occurred in the Adirondack Lakes in upstate New York. This region, which features almost 3,000 lakes, began showing signs of acidification as early as the 1970s, primarily due to acid rain caused by industrial emissions of sulfur dioxide (SO₂) and nitrogen oxides (NOₓ).[3] These pollutants, emitted by power plants and industrial facilities in the Midwestern United States, were carried by winds to the Adirondack region, where they deposited into water bodies and surrounding soils, lowering the pH levels of lakes and streams.[25] The acidification of these waters led to a significant decline in aquatic biodiversity, including the disappearance of fish and crustaceans species.[26]
Efforts to reduce acid rain through the Clean Air Act Amendments of 1990, which targeted reductions in SO₂ and NOₓ emissions, have led to some recovery in the Adirondack Lakes. Monitoring data shows improvements in water quality, although many ecosystems remain vulnerable due to the long-lasting effects of acid deposition on soils and watersheds.[27] This case demonstrates how regulatory interventions, such as the Clean Air Act Amendments, have played a role in addressing the anthropogenic causes of freshwater acidification, though studies show that ecological recovery remains challenging due to the long-term impacts of acid deposition.[28]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.