Loading AI tools
From Wikipedia, the free encyclopedia
In chemistry, the dodecahedral molecular geometry describes the shape of compounds where eight atoms or groups of atoms or ligands are arranged around a central atom defining the vertices of a snub disphenoid (also known as a trigonal dodecahedron). This shape has D2d symmetry and is one of the three common shapes for octacoordinate transition metal complexes, along with the square antiprism and the bicapped trigonal prism.[1][2]
| Dodecahedral molecular geometry | |
|---|---|
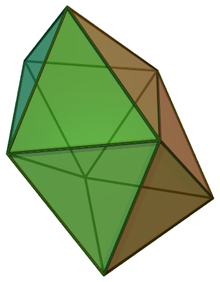 | |
| Examples | Mo(CN)4− 8 |
| Point group | D2d |
| Coordination number | 8 |
| μ (Polarity) | 0 |
One example of the dodecahedral molecular geometry is the Mo(CN)4−
8 ion.[2]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.