Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
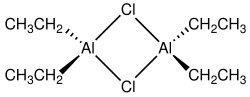
Diethylaluminium chloride, abbreviated DEAC, is an organoaluminium compound. Although often given the chemical formula (C2H5)2AlCl, it exists as a dimer, [(C2H5)2AlCl]2 It is a precursor to Ziegler-Natta catalysts employed for the production of polyolefins. The compound is also a Lewis acid, useful in organic synthesis. The compound is a colorless waxy solid, but is usually handled as a solution in hydrocarbon solvents. It is highly reactive, even pyrophoric.[2]
 | |
| Names | |
|---|---|
| IUPAC name
Chlorodiethylalumane | |
| Other names
Chlorodiethylaluminium | |
| Identifiers | |
3D model (JSmol) |
|
| 4123259 | |
| ChemSpider | |
| ECHA InfoCard | 100.002.253 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
| UN number | 3394 |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C8H20Al2Cl2 | |
| Molar mass | 241.11 g·mol−1 |
| Appearance | Colorless liquid[1] |
| Density | 0.96 g/cm3[1] |
| Melting point | −74 °C (−101 °F; 199 K)[1] |
| Boiling point | 125 to 126 °C (257 to 259 °F; 398 to 399 K) at 50 mmHg |
| Reacts[1] | |
| Vapor pressure | 3 mmHg (at 60 °C) |
| Hazards | |
| GHS labelling: | |
  | |
| Danger | |
| H225, H250, H260, H261, H314 | |
| P210, P222, P223, P231+P232, P233, P240, P241, P242, P243, P260, P264, P280, P301+P330+P331, P302+P334, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P335+P334, P363, P370+P378, P402+P404, P403+P235, P405, P422, P501 | |
| NFPA 704 (fire diamond) | |
| Flash point | −18 °C (0 °F; 255 K) |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Compounds of the empirical formula AlR2Cl (R = alkyl, aryl) usually exist as dimers with the formula (R2Al)2(μ-Cl)2. The bridging ligands (indicated by "μ-") are halides, not the organic substituents. The aluminium adopts a tetrahedral geometry. Each Al(III) center follows the octet rule.[3][4] In contrast, triethylaluminium and trimethylaluminium feature bridging alkyl groups and these compounds violate the octet rule.
Diethylaluminium chloride can be produced from ethylaluminium sesquichloride, (C2H5)3Al2Cl3, by reduction with sodium:[5]
It is also obtained from the reaction of triethylaluminium with hydrochloric acid:
Reproportionation reactions can also be used:
Diethylaluminium chloride and other organoaluminium compounds are used in combination with transition metal compounds as Ziegler–Natta catalysts for the polymerization of various alkenes.[6]
As a Lewis acid, diethylaluminium chloride also has uses in organic synthesis. For example, it is used to catalyze the Diels–Alder and ene reactions. Alternatively, it can react as a nucleophile or a proton scavenger.[2]
Diethylaluminium chloride is not only flammable but pyrophoric.
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.