Bisphenol
Class of chemical compounds From Wikipedia, the free encyclopedia
Class of chemical compounds From Wikipedia, the free encyclopedia
The bisphenols (/ˈbɪsfɪnɒl/) are a group of industrial chemical compounds related to diphenylmethane; commonly used in the creation of plastics and epoxy resins.[1][2][3] Most are based on two hydroxyphenyl functional groups linked by a methylene bridge. Exceptions include bisphenol S, P, and M. "Bisphenol" is a common name; the letter following denotes the variant, which depends on the additional substituents. Bisphenol A is the most popular representative of the group, with millions of metric tons produced globally in the past decade, often simply called "bisphenol".[3][4][5]
| Structural formula | Name | CAS | Reactants | |
|---|---|---|---|---|
| Bisphenol A | 80-05-7 | Phenol | Acetone | |
 | Bisphenol AP | 1571-75-1 | Phenol | Acetophenone |
 | Bisphenol AF | 1478-61-1 | Phenol | Hexafluoroacetone |
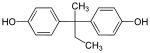 | Bisphenol B | 77-40-7 | Phenol | Butanone |
 | Bisphenol BP | 1844-01-5 | Phenol | Benzophenone |
 | Bisphenol C | 79-97-0 | o-cresol | Acetone |
 | Bisphenol C 2 | 14868-03-2 | Phenol | Chloral |
| Bisphenol E | 2081-08-5 | Phenol | Ethanal | |
| Bisphenol F | 620-92-8 | Phenol | Formaldehyde | |
 | Bisphenol G | 127-54-8 | 2-Isopropylphenol | Acetone |
| Bisphenol M | 13595-25-0 | |||
 | Bisphenol S | 80-09-1 | Phenol | Sulfur trioxide |
 | Bisphenol P | 2167-51-3 | ||
 | Bisphenol PH | 24038-68-4 | 2-Phenylphenol | Acetone |
 | Bisphenol TMC | 129188-99-4 | Phenol | 3,3,5-Trimethylcyclohexanone |
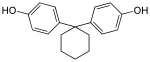 | Bisphenol Z | 843-55-0 | Phenol | Cyclohexanone |
 | Dinitrobisphenol A | 5329-21-5 | Bisphenol A | Nitric acid |
 | Tetrabromobisphenol A | 79-94-7 | Bisphenol A | Bromine |
Bisphenols A (BPA), F (BPF) and S (BPS) have been shown to be endocrine disruptors, potentially relating to adverse health effects.[3][6] Due to its high production volumes, BPA has been characterised as a "pseudo-persistent" chemical,[7] leading to its spreading and potential accumulation in a variety of environmental matrices, even though it has a fairly short half-life.[8]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.