Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
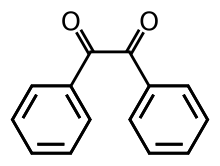
Benzil (i.e. Bz2, systematically known as 1,2-diphenylethane-1,2-dione) is the organic compound with the formula (C6H5CO)2, generally abbreviated (PhCO)2. This yellow solid is one of the most common diketones. Its main use is as a photoinitiator in polymer chemistry.[4]
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Diphenylethanedione | |
| Systematic IUPAC name
1,2-Diphenylethane-1,2-dione | |
| Other names
Diphenylethane-1,2-dione Benzil Dibenzoyl Bibenzoyl Diphenylglyoxal | |
| Identifiers | |
3D model (JSmol) |
|
| 608047 | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.004.689 |
| EC Number |
|
PubChem CID |
|
| RTECS number |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C14H10O2 | |
| Molar mass | 210.232 g·mol−1 |
| Appearance | yellow crystalline powder |
| Density | 1.23 g/cm3, solid (1.255 g/cm3, x-ray) |
| Melting point | 94.0 to 96.0 °C; 201.2 to 204.8 °F; 367.1 to 369.2 K |
| Boiling point | 346.0 to 348.0 °C; 654.8 to 658.4 °F; 619.1 to 621.1 K |
| insoluble | |
| Solubility in ethanol | soluble |
| Solubility in diethyl ether | soluble |
| Solubility in benzene | soluble |
| -118.6·10−6 cm3/mol | |
| Structure | |
| P31,221[1] | |
| 3.8 D[2] | |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
Main hazards |
Irritant |
| GHS labelling: | |
 | |
| Warning | |
| H315, H319, H335 | |
| P261, P264, P271, P280, P302+P352, P304+P340, P305+P351+P338, P312, P321, P332+P313, P337+P313, P362, P403+P233, P405, P501 | |
| NFPA 704 (fire diamond) | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) |
>3 g/kg (mouse, oral)[3] |
| Related compounds | |
Related diketones |
diacetyl |
Related compounds |
benzophenone glyoxal bibenzil |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
The compound's most noteworthy structural feature is the long carbon-carbon bond of 1.54 Å, which indicates the absence of pi-bonding between the two carbonyl centers. The PhCO centers are planar, but the pair of benzoyl groups are twisted with respect to the other with a dihedral angle of 117°.[5] In less hindered analogues (glyoxal, biacetyl, oxalic acid derivatives), the (RCO)2 group adopts a planar, anti-conformation.
Most benzil can be used as a photoinitiator in the free-radical curing of polymer networks. It absorbs ultraviolet radiation at a wavelength of 260 nm, leading to decomposition with formation of free-radical species and formation of cross-links within the material. However, it is a relatively poor photoinitiator, and is seldom used. It undergoes photobleaching, which allows the curing light to reach deeper layers of the material on longer exposure.[6] Acetal derivatives, such as 2,2-dimethoxy-2-phenylacetophenone, have better properties for this application.[6]
Benzil is a potent inhibitor of human carboxylesterases, enzymes involved in the hydrolysis of carboxylesters and many clinically used drugs.[7]
Benzil is a standard building block in organic synthesis. It condenses with amines to give diketimine ligands. A classic organic reaction of benzil is the benzilic acid rearrangement, in which base catalyses the conversion of benzil to benzilic acid. This reactivity is exploited in the preparation of the drug phenytoin. Benzil also reacts with 1,3-diphenylacetone in an aldol condensation to give tetraphenylcyclopentadienone.
Benzil is prepared from benzoin, for example with copper(II) acetate:[8]
Other suitable oxidizing agents such as nitric acid (HNO3) are used routinely.
Iron(III) chloride (FeCl3) can be used as an inexpensive catalyst for this chemical conversion.[9]
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.