Chemical compound From Wikipedia, the free encyclopedia
Barium carbide (also referred to as barium ethynediide or barium acetylide)[1] is a chemical compound in the carbide family having the chemical formula BaC2.[2]
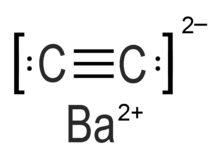 | |
| Names | |
|---|---|
| IUPAC name
Barium ethynediide | |
| Other names
Barium acetylide | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
| EC Number |
|
| |
| |
| Properties | |
| BaC2 | |
| Molar mass | 161.35 g/mol |
| Appearance | black crystalline solid |
| Density | 3.75 g/cm3 |
| Related compounds | |
Other cations |
Calcium carbide; Strontium carbide |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
Barium carbide can be synthesized as an impure compound by reducing barium carbonate powder with metallic magnesium in the presence of carbon.[3] Barium carbide can also be made by reducing carbon dioxide with hot barium metal at 600°C.[4] These methods are used because of their high yield, and because the carbide is used to make acetylene. It can also be prepared by heating a barium amalgam and carbon powder mixture in a hydrogen current. The pure compound is prepared by reducing barium oxide with carbon at high temperature.[5]
Barium carbide reacts similarly to calcium carbide,[6] but it's more fusible. When exposed to extreme heat, the barium will evaporate leaving behind crystals of graphite. It can also absorb the carbon in a solution at high temperature.[5]
Barium carbide can cause damage to the GI tract and irritation in the skin and eyes.[1]
Seamless Wikipedia browsing. On steroids.