Loading AI tools
Chemical compound From Wikipedia, the free encyclopedia
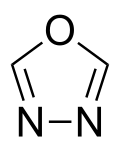
1,3,4-Oxadiazole is a nitrogen and oxygen containing heterocycle, and one of the four isomers of oxadiazole.[1][2]
 | |
| Names | |
|---|---|
| Preferred IUPAC name
1,3,4-Oxadiazole | |
| Identifiers | |
3D model (JSmol) |
|
| ChemSpider | |
PubChem CID |
|
| UNII | |
CompTox Dashboard (EPA) |
|
| |
| |
| Properties | |
| C2H2N2O | |
| Molar mass | 70.051 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).
| |
1,3,4-Oxadiazole itself is not commonly used in organic chemistry, but many of its derivatives are important. For example, raltegravir is an HIV drug which contains an 1,3,4-oxadiazole ring. Other pharmaceutical drugs containing the 1,3,4-oxadiazole ring include fenadiazole, zibotentan, and tiodazosin.
1,3,4-Oxadiazole derivatives can be synthesized in a variety of ways.[3] One pathway is from oxidation of tetrazoles in the presence of aldehydes.[4] Similarly, the reaction of tetrazoles with acyl chlorides provides oxadiazoles.[5] Both methods involve the release of N2.
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.