製備
三氟化鋯可以由氫化的鋯和氟化氫與氫氣的混合物在750 °C下反應而成。[1]
它也可以由(NH4)2ZrF6在650 °C下被氫氣還原而成。[2]
性質
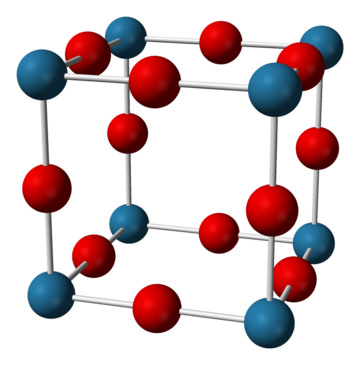
三氟化鋯是藍灰色固體,在300 °C下分解。它難溶於熱水、易溶於熱酸、不溶於氫氧化鈉溶液和氨水。三氟化鋯的晶體結構是三氧化錸結構。[1]
參考資料
Wikiwand in your browser!
Seamless Wikipedia browsing. On steroids.
Every time you click a link to Wikipedia, Wiktionary or Wikiquote in your browser's search results, it will show the modern Wikiwand interface.
Wikiwand extension is a five stars, simple, with minimum permission required to keep your browsing private, safe and transparent.