 通用名药物
通用名药物Bioequivalence. Approved Drug Products with Therapeutic Equivalence Evaluations 29th Edition. U.S. Food and Drug Administration Center for Drug Evaluation
 恩替卡韦
恩替卡韦(原始内容存档于2013-03-24). Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. U.S. Food and Drug Administration (FDA). [2015-08-29]
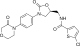 利伐沙班
利伐沙班2017]. (原始内容存档于2018-06-16). Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. www.accessdata.fda.gov. [April 24, 2019].
 环丙沙星
环丙沙星Google Patents. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations N019537. U.S. Food and Drug Administration (FDA). [2014-01-05]
 瑞芬太尼
瑞芬太尼Feb. 1999. US Food and Drug Administration. (2010). Orange book: approved drug products with therapeutic equivalence evaluations. Silver Spring, MD: US




