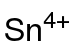氢氧化锡
化合物 来自维基百科,自由的百科全书
制备
将四氯化锡用冰水稀释,得到氧氯化锡(SnOCl2)的溶液,在溶液中逐滴加入氨水,可以得到氢氧化锡的水合物。[2]
- SnOCl2 + 2 NH3·H2O → Sn(OH)4 + 2 NH4Cl
性质
氢氧化锡在100 °C的水中回流,发生分解,生成二氧化锡:[2]
- Sn(OH)4 → SnO2 + 2 H2O
在该反应中,是否使用超声对反应也会产生影响。[3]此外,电化学研究表明,Sn(OH)4超过其饱和浓度时,有生成热力学上更稳定的SnO2·xH2O的趋势。[4]
参考文献
参见
Wikiwand - on
Seamless Wikipedia browsing. On steroids.